Galectins are saccharide-binding proteins with characteristic amino acid sequences in the carbohydrate recognition domain. Many members of the galectin family also show two other features: they reside both inside as well as outside of cells and they bind multiple partners through protein-protein interactions. Inside cells, some examples of non-carbohydrate ligands of various galectins include: (a) prototype galectin-1 binds to oncogene H-Ras and transcription factors OCA-B and TFII-I; (b) tandem-repeat type galectin-8 binds to other galectins such as galectin-9, the autophagy receptor NDP52, and tripartite motif protein TRIM5α; and (c) the NH2-terminal domain of chimera type galectin-3 binds to components of the endosomal sorting complex required for transport such as Tsg101 and to ribonucleoprotein complexes such as hnRNP A2B1 while the COOH-terminal domain binds to the apoptosis repressor Bcl-2, tripartite motif protein TRIM16, and transcription factors OCA-B, TFII-I, and β-catenin. The present article aims to summarize selected studies that highlight the promiscuity of intracellular galectins and several intriguing questions raised by these findings. ...and more
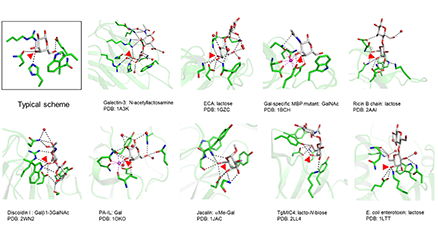
The natural ligands of galectins are mainly glycoproteins and to some extent possibly glycosphingolipids. The binding affinity and specificity of galectins for small free saccharides has been studied extensively, but not the binding of saccharides when attached to natural glycoproteins or glycolipids. Galectins often bind a glycoprotein or cell surface with higher apparent affinity than the corresponding glycans when tested as free oligosaccharides.. Here I will argue and provide evidence that this may not be due mainly to multivalent interactions, but instead that extended binding sites on the galectin-carbohydrate recognition domain (CRD) providing higher monovalent affinity makes important contributions. Such monovalent interactions can also be highly selective, where the selectivity comes both from the structure of the glycan bound, and protein parts near where it is attached. Multivalency is instead critical for galectins ability to cross-link ligands in various ways, a key part of their function. The cross-linking depends on the monovalent selectivity, but can also add an additional layer of selectivity and contribute to affinity. The main focus will be on galectins-1 and -3, but other galectins are also included. The equally interesting interaction of galectins with non-glycosylated protein ligands intracellularly is not covered here. ...and more

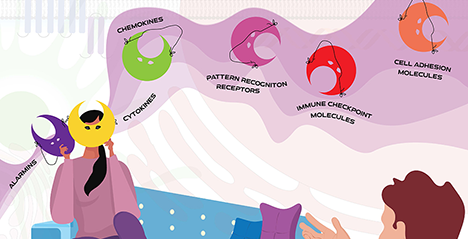
Multiple personality disorder is a psychiatric condition characterized by the spontaneous generation of alternate versions of self. The personalities have distinctive styles of expressing themselves, and they often possess separate names, living locations, and lifestyles. The transition from one personality to another is commonly triggered by environmental and interpersonal factors. Unlike many cellular molecules and pathways, which are often specialized, this concept could be certainly applied to galectins, an evolutionarily conserved family of glycan-binding proteins with multifunctional roles in immunity. These proteins often shuttle between different intracellular compartments (nucleus, cytoplasm, and organelles) and are released to the extracellular milieu, where they acquire different roles in response to diverse microenvironmental stimuli, including hypoxia, nutrient availability, intracellular and extracellular pH, cytokine milieu and the presence of proinflammatory or immunosuppressive signals. Within the immune system, galectins can elicit a wide array of important functions that tailor both innate and adaptive responses, playing key roles in shaping the choreography of immune cells in health and disease. Interestingly, the same galectin can function as a cytokine, chemokine, cell adhesion molecule, immune checkpoint molecule, danger-associated molecular pattern, or growth factor depending on different cellular programs, including activation, differentiation, and trafficking, or during pathologic conditions, such as pathogen invasion, autoimmune inflammation, fibrosis and cancer. ...and more
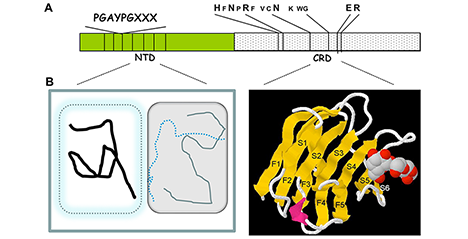
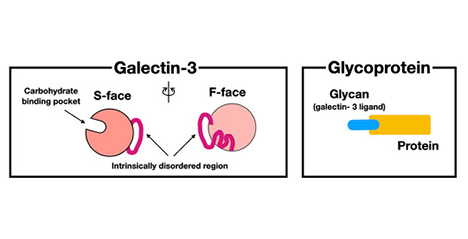
Galectin-3 is one of the most extensively studied members of the galectin family along with galectin-1. Galectin-3 is involved in a wide variety of functions and places, in vitro in both intracellular and extracellular spaces and in vivo in various organs. As mentioned in Part 1, galectin-3 contains an “intrinsically disordered peptide region” with repeated peptide sequences, and the total length of these peptides is almost the same as the carbohydrate-binding domain (CBD) at the C-terminus (Figure 3) . Notably, this region is not found in other animal lectins. Most of the reported activities of galectin-3 require this intrinsically disordered region. Galectin-3, which usually exists as a monomer, oligomerizes in a manner that is dependent on this region after binding to its glycan ligand. Therefore, the activity of galectin-3 is thought to be regulated primarily by intrinsically disordered region-dependent oligomerization. In Part 2, I would first like to delve deeper into the mechanism by which the unique intrinsically disordered region is involved in the oligomerization of galectin-3 by referring to the latest reports. Then, I will explore the relationship between the oligomerization of galectin-3 and its diverse functions. ...and more
Galectin-3 is structurally distinct from other galectin family members by having not only a lectin domain (carbohydrate-binding domain: CBD) with an affinity for β-galactoside at the C-terminus, but also a non-lectin region of a similar length to the CBD at the N-terminus. Most of the non-lectin region comprises a proline-, glycine-, and tyrosine-rich peptide consisting of 9–13 amino acids, which is repeated 9–12 times. It is an “intrinsically disordered peptide region” that does not form any specific secondary and tertiary structures. Previous studies have indicated that glycan-bound galectin-3 may be involved in liquid-liquid phase separation (LLPS) using this intrinsically disordered N-terminal peptide region. ...and more
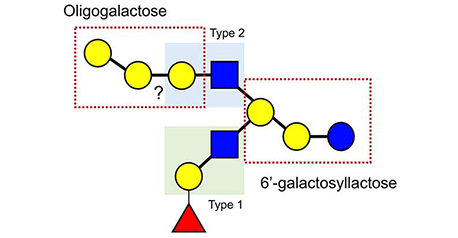
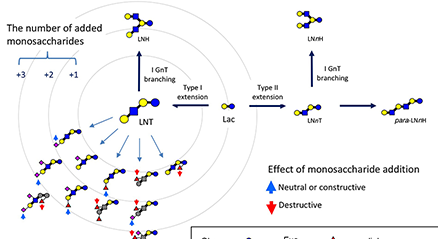
Human milk contains 12~13 g/L of oligosaccharides, of which around 250 varieties have been separated and more than 160 structures have been characterized. Human milk oligosaccharides (HMOs) have been classified into 20 series based on the core structures, which contain neither fucose nor sialic acid, and the biosynthetic pathways of these structures have been proposed based on the activities of β-1-3-N-acetylglucosaminyltransferase (iGnT), β-1-6-N-acetylglucosaminyltransferase (IGnT), β-1-3-galactosyltransferase (β3GalT) as well as β-1-4-galactosyltransferase (β4GalT). Around 50 varieties of oligosaccharides have been also separated and characterized from bovine colostrum, and the biosynthetic pathways of their core structures have been proposed as well. ...and more
Galectins are a family of small soluble glycan-binding proteins, implicated in a number of cellular functions and attracting attention as diagnostic and therapeutic targets for diseases such as inflammation, fibrosis and cancer. However, they occur in a bewildering number of functional contexts, intracellular and extracellular, low and high concentrations, slow and fast, low or high specificity, and there is no clear statement like: “this is the function of galectins, or even any particular galectin”. Here I will give an overview of galectins in the form of categorical statements to help raise interesting outstanding questions. The statements may not be true in all detail, but, I believe, are largely true and help make the questions clearer. ...and more
In the preceding chapter (Part 1), the authors described the origin of galectins while focusing on the point that galectins are essentially cytosolic proteins1. In this chapter, one of the authors (J.H.) hypothesizes that the presence of carbohydrates is prerequisite to the generation of lectins that recognize them as counterpart molecules, and that the presence of galactose (the carbohydrate ligand of galectin) is prerequisite to the generation of galectin. Clearly, the question is: what is the origin of galactose? In this chapter and as previously proposed by Hirabayashi (for details, see refs. 1-4), a hypothesis regarding the “late arrival of galactose” and the origin of saccharides is presented. ...and more
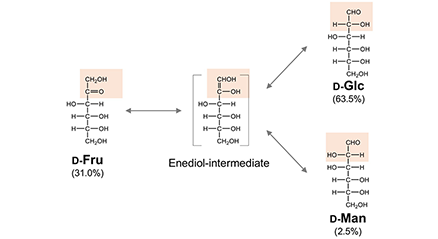
Galectins are characterized as a group of evolutionarily related proteins having a conserved unique sequence with the ability to bind galactose (rigorously to bind β-galactoside). To date, their diverse biological distributions and functions have been described; however, the origin of galectin has only scarcely been discussed. Curiously, all of them so far investigated lack a signal sequence required for secretion, while they are assumed to function in extracellular spaces, where a vast amount of galectin-binding glycans exist. In fact, regardless of biological species, all the galectins are biosynthesized as “cytosolic proteins”. This suggests that they have intrinsic functions in the cytosol. In this chapter (Part 1), we describe the origin of galectins in light of their cytosolic features. In the following chapter (Part 2), We attempt to discuss the most fundamental issues of galactose, to which all galectins bind, by referring to the origins of monosaccharides and their chirality. ...and more
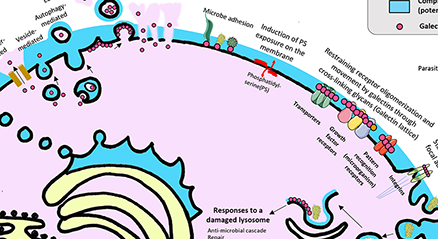
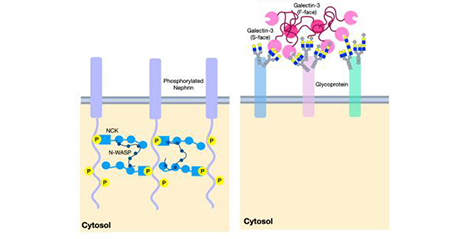
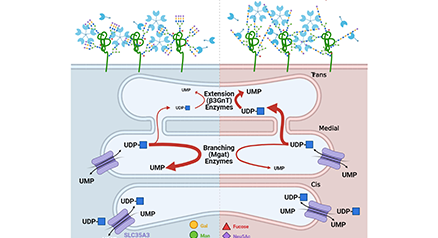
The galectin lattice is a multi-valent interaction of galectins with glycoproteins at the cell surface that displays rapid exchange of binding partners with properties of liquid-liquid phase transitions, thereby acting as an intermediary between freely diffusing glycoproteins and stable complexes in the membrane. The galectin lattice (i) regulates flow of receptors and solute transporters to coated-pit endocytosis and/or caveolin domains, and (ii) promotes turnover of cell-cell contacts such as immune synapses and focal adhesion complexes. Metabolic regulation of UDP-GlcNAc supply to Golgi N-glycan remodeling regulates glycoprotein affinities for galectins –and in turn, trafficking and presentation at the cell surface. The lattice model has been validated in immune regulation, cancer progression and glucose homeostasis in mice. Here we review the interactions of metabolism, galectins and glycoprotein ligands as well as the utility of this model to predict and treat inflammation and autoimmunity. ...and more
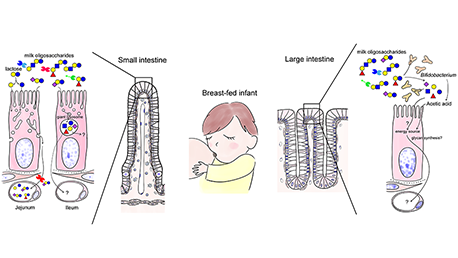
The binding affinity of lectins for glycans is generally displayed at the µM level; however, milk contains more than 10 mM oligosaccharides and 100 mM lactose. Such an astonishingly high concentration of glycans present in milk could inhibit the binding of most endogenous lectins to their glycan ligands. Then, many questions naturally arise, for example: what is the biological significance of the inhibitory activity? How high are the milk oligosaccharide concentrations in the digestive tract, and what is the pharmacokinetics of milk oligosaccharides in the body?
In this spin-off version, we try to present our guesses or “daydream” – our extended speculation – and discuss about how milk oligosaccharides are used once they enter the body, what roles they may play, and what their relationship may be to the function of galectins, which are abundant in the digestive tract. In addition, we reconsider the reason why mammals have acquired the ability to biosynthesize lactose and use it as a nutrient source for babies, which is said to be the “key to the evolutionary success of mammals”. ...and more
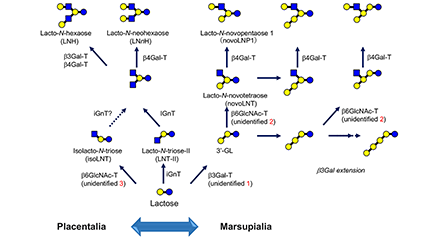
In the main chapter of this series, “Milk oligosaccharides and galectins” (Glycoforum. 2021 Vol. 24 (2), A3), we commented that rat milk includes largely 3’-sialyllactose (3’-SL) and 6’-sialyllactose (6’-SL), while that of another rodent species, the lowland paca (Cuniculus paca), possesses more complex structures of milk oligosaccharides. This observation inspires us to postulate that the milk oligosaccharides of experimental animals are much simpler than those of wild animals. However, it was recently reported that rat and mouse milks contain a small amount of more complex oligosaccharides, such as sulfo 3’-sialyllactose. Here, we draw attention to another unsolved question as a theme for this second spin-off version: how is the structural diversity of milk oligosaccharides defined?...and more
This chapter is a “spin-off” version of “Milk oligosaccharides and Galectins” by Urashima and Hirabayashi, published in the main part of the Galectin series (Vol. 24 [2], A3). Here, we chose a few topics that could not be fully dealt with in the main chapter, thereby aiming to advance discussions about important, but unsolved questions of milk oligosaccharides, in a free and active forum with the extended members. We hope you will enjoy this new forum style. ...and more
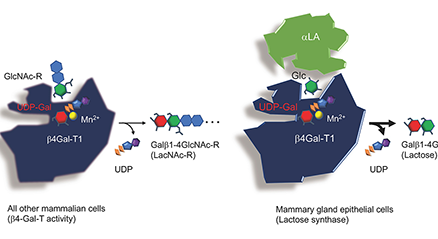
Mammalian milk or colostrum usually contains a variety of milk oligosaccharides in addition to lactose (Galβ1-4Glc) as the predominant saccharide. Almost all of them have a lactose unit at their reducing ends and also contain galactose (Gal), N-acetylglucosamine (GlcNAc), fucose (Fuc), N-acetyl or N-glycolylneuraminic acid (Neu5Ac and Neu5Gc, respectively), or N-acetylgalactosamine (GalNAc) residues. For example, human milk contains more than 250 varieties of oligosaccharides at a concentration of 12–13 g/L (see Note 1). Around 170 of the more than 250 chemical structures have been characterized to date.1 In this chapter of the series, the potential utilization of milk oligosaccharides is discussed as a unique research tool to solve the unanswered questions related to galectins. ...and more
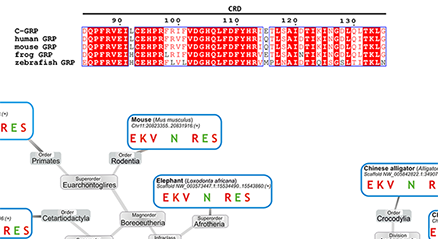
Lectins have a central role in translating glycan-encoded information into bioactivity. They are classified according to the fold of their carbohydrate recognition domain (CRD). This structural unit is proposed to be capable to do (much) more than to interact with cognate glycan(s). As outlined for the proof-of-principle case of the galectin CRD, distinct sequence elements appear capable to extend a CRD’s functionality profile: they can implement molecular switches for activity, conformation and quaternary structure, even establish complementary regions for other types of binding partners, letting the CRD gain an unsuspected multifunctionality. By further testing and validating these suggestions, answers may be provided for major unresolved questions, e.g. why galectins belong to the small set of secreted proteins without a signal sequence. This case study teaches the salient lesson of the possibility for a CRD to consist of various structural elements beyond that binding the sugar. Of potential relevance for public health, the idea of a gene capture from the host as origin of viral adhesins that have a galectin-like fold (incl. coronaviruses such as SARS-CoV-2) may inspire innovative modalities to counter pandemic threats. ...and more
Galectin is a well-known molecular entity but remains difficult to understand. Although it appears on a wide variety of stages, it is seldom the main actor; its role seems to be supporting but indispensable. It acts with a wide variety of partners whatever their identity (protein, lipid, or proteoglycan) or position, as long as they are wearing a favorite costume (glycan). Therefore, almost all (more than 10,000) extracellular proteins can be its partners, although its relationships with them are not so intimate. This wide and shallow sociality makes the essential nature of galectin difficult to understand. With these aspects in mind, I would like to review some basic points concerning galectin and its weak interactions. ...and more

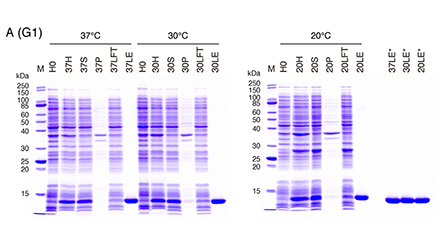
This note describes unpublished data regarding the expression (in E. coli) and purification of human galectins. I hope that the data and know-how provided below will be useful for researchers (especially for those having limited experience in biochemistry) who are not acquainted with the galectin family and have a need to prepare recombinant galectins. ...and more
A brand new Glycoforum® genre series related to Galectins will be started, combined with publication of many detailed experimental protocols for galectin production. As editors of this series we hope to bring unique perspectives to the field of galectins and glycoscience. ...and more