
Sachiko Sato
Research Centre for Infectious Diseases, Faculty of Medicine, Laval University, Quebec City, Canada. Ph.D, Pharmaceutical science
Sachiko Sato graduated from Faculty of Pharmaceutical Science, Chiba University. She joined as postgraduate student in the laboratory of Dr. Akira Kobata, the Institute of Medical Science, the University of Tokyo, Japan in 1987. She also worked in the laboratory of Dr. R. Colin Hughes, MRC: National Institute for Medical Research in London, UK, where she first encountered a cytosolic mammalian lectin, now called galectin-3. She obtained her Ph. D. from the University of Tokyo in 1994. As postdoctoral fellow in the laboratory of Dr. Ron Kopito, Stanford University, she was involved in the work on cystic fibrosis. She became principal investigator of the laboratory of glycobiology in Research Center for Infectious Diseases, and assistant professor of the Faculty of Medicine, Laval University, Quebec, Canada in 1999 and is full professor since 2010. She is also director of the Bioimaging platform since 2003.
Galectin-3 is structurally distinct from other galectin family members by having not only a lectin domain (carbohydrate-binding domain: CBD) with an affinity for β-galactoside at the C-terminus, but also a non-lectin region of a similar length to the CBD at the N-terminus. Most of the non-lectin region comprises a proline-, glycine-, and tyrosine-rich peptide consisting of 9–13 amino acids, which is repeated 9–12 times1. It is an “intrinsically disordered peptide region” that does not form any specific secondary and tertiary structures. Previous studies have indicated that glycan-bound galectin-3 may be involved in liquid-liquid phase separation (LLPS) (Note 1) using this intrinsically disordered N-terminal peptide region2–6.
Note 1: LLPS is a well-established field in physical chemistry, but it was Hyman’s research group that proposed (or started) linking it to life phenomena55. Until recently, LLPS was studied in relation to unravelling intracellular phenomena and was not thought to occur on the cell membrane in the same way. In 2015, Dennis first proposed that the function of galectins on the plasma membrane was related to LLPS2, and Huang’s group in Taiwan first presented experimental data supporting this in 20173.
The space in which LLPS occurs is often referred to as a membraneless cellular organelle. LLPS involves the separation of multiple substances dissolved in a solution into two phases without mixing, causing specific biological substances to accumulate in spaces at high concentrations, in the absence of a membrane. Moreover, these spaces can be rapidly formed or dissolved by subtle environmental changes such as temperature, salt concentration, pH, and the presence of small molecules. Various biological phenomena are thought to be efficiently generated and controlled in condensed LLPS spaces created at specific times and places. LLPS requires an accumulation of multiple weak or fragile interactions through intrinsically disordered peptide regions, which shares certain biochemical features unique to galectin-3: (i) both the intrinsically disordered region of galectin-3 and its oligosaccharide ligands are flexible and have no specific structure, (ii) the interaction between galectin-3 and its ligands is “weak and often ambiguous” 7,8, and (iii) oligomerization occurs upon binding to oligosaccharides. In addition, the CBDs of galectin-3 interact with each other as well as with the CBDs of different galectins9, allowing galectin-3 to serve as a scaffold in which other galectins may also participate in the LLPS. The LLPS may therefore provide a perspective for furthering our understanding of the intracellular and extracellular functions of the cytoplasmic lectin, galectin-3, as well as leading to the point7,8 that "Strong and rigorous binding is not the only important Issue." In this forum, I discuss these points biochemically and biologically, with speculations and hypotheses.
Regarding the possible induction of LLPS by galectin-3, we cannot avoid discussing its unique oligomerization, which notably remains a mystery. In addition, no comprehensive review has yet been published to date. In this article (Part 1), I therefore discuss previous research into the oligomerization of galectin-3, and also consider its oligomerization in relation to LLPS, including the hot topic of LLPS as a membraneless organelle and the limitations of current LLPS research. In Part 2, I introduce recent research on the involvement of galectin-3 in LLPS, the relationship between LLPS and the intracellular and extracellular functions of galectin-3, and a possible link with the functions of other galectins. In writing this forum, I searched for papers focusing on LLPS, but emphasize that I have not covered all publications on the oligomerization of galectin-3, which has been investigated for more than 30 years.
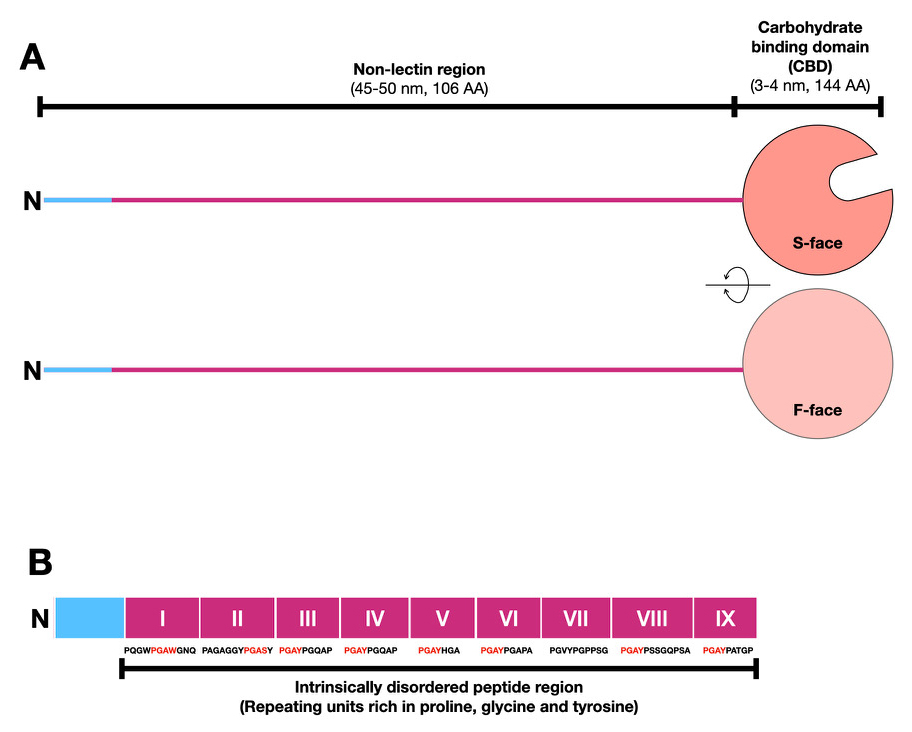
Members of the galectin family of β-galactoside-binding proteins are divided structurally into three types10. Prototype proteins consist of only a CBD with a tertiary structure resembling like a jelly-roll cake, whereas the tandem-repeat type has two CBDs connected via a relatively short peptide. Chimera-type galectins consist of a CBD at the C-terminal and a non-lectin domain (usually called an N-terminal domain) with a similar molecular weight to the CBD (Fig. 1A). In vertebrates, galectin-3 is the only chimeric type, whereas there are several prototype and tandem-repeat type proteins1. At that time (1999), this taxonomic name was proposed assuming the existence of chimeric galectins with various N-terminal non-lectin regions other than galectin-310. However, it turns out that it is not the case (personal communication with Dr. Hirabayashi). Why are there not any other chimera-type galectins besides galectin-3?
The CBD of galectin-3 has two faces: the β-sheet plane with a sugar-binding pocket consisting of 6 β-strands is called the S-face, and the opposite plane consisting of 5 β-strands is called the F-face (Fig. 1A).
The non-lectin region of galectin-3, which is lacking in other galectins (Fig. 1B), consists of a peptide sequence of 18 amino acid residues at the N-terminus followed by 7–14 repeats of a sequence of 7–12 amino acid residues consisting mainly of proline (P), glycine (G), and tyrosine (Y). This repeating region does not form secondary or tertiary structures. Such a polypeptide region that does not have any stable tertiary structure is called an “intrinsically disordered peptide region”. The galectin-3-specific N-terminal region is usually called the N-terminal non-lectin domain; however, the term “domain” should only be used for polypeptides that have formed secondary or tertiary structures. Thus, in this forum, I will therefore use the term “region” instead of domain. In addition, I refer to the proline-glycine-tyrosine-rich repeated region within the N-terminal non-lectin region as the intrinsically disordered region (Fig. 1B).
Galectin-3 is the most-studied member of this family, along with galectin-111. At the intracellular level, it plays an important role in autophagy12, 13, is involved in cilia function14, 15, binds RNA, has RNA-splicing activity16, 17, and is known to regulate Ras activity18. Extracellularly, it induces clathrin-independent endocytosis at low concentrations19-21 and, at higher concentrations, forms galectin lattices, which are involved in regulating the dynamics of cell surface glycoproteins22–26. It increases the efficiency of muscle regeneration27. In vivo, galectin-3 is involved in the induction or regulation of the initial stage of innate immunity11, 28 and the suppression of adaptive immune responses through modulation of the threshold29–31, as well as augmenting cancer metastasis and tissue fibrosis32, 33. It has been suggested that many (but not necessarily all) of these activities require both the binding of its CBD to the ligands and oligomerization. However, as detailed below, this lectin normally exists as a monomer, and how then does it become an oligomer?
Galectin-3 has only one CBD per molecule, unlike many prototype galectins such as galectin-1, which forms a dimer, and tandem-repeat type galectins, such as galectin-9, which has two CBDs per molecule. In the absence of ligands, galectin-3 exists as a monomer (Note 2) and does not form an oligomer, including a dimer. However, galectin-3 shows hemagglutinating activity and has thus been assumed to form a dimer or oligomer after binding to its oligosaccharide ligands. Despite intermittent but extensive studies on the molecular mechanism of its oligomerization over the past 30 years, the mechanism has still not been fully elucidated. I will thus start by describing the findings on the oligomerization of galectin-3 before 2000, and will then introduce the mechanisms proposed in recent research using new approaches.
Note 2: It has been confirmed that galectin-3 is a monomer even at high concentrations (20 µM)35.
In 1992, Liu’s group found that the non-lectin region of galectin-3 is sensitive to collagenase. They used this characteristic to prepare a CBD without the non-lectin region and compared its binding characteristics to full-length galectin-3. They found that full-length galectin-3 binds to immobilized oligosaccharides in a concentration-dependent manner at low concentrations (below 0.3 µM), but binds cooperatively at concentrations above approximately 1 µM (positive cooperative binding (Note 3) ). In contrast, the CBD does not demonstrate this positive cooperative binding. This was the first report to reveal that this unusual type of lectin binding requires the presence of a non-lectin region34. This result implies that oligomerization of galectin-3 may occur after binding to immobilized oligosaccharides.
Note 3: Positive cooperative binding: unlike normal interactions in which individual elements act independently, cooperative binding is a phenomenon in which elements (here, galectin-3) act dependently on each other (here, binding to glycans).
In 1993, Barondes’ group also reconfirmed that positive cooperative binding occurs at galectin-3 concentrations of 0.3 µM or above. Their results suggest that this phenomenon does not require the protein portion of the glycoprotein and does not occur when a small soluble sugar ligand is used35. The sensitivity of cooperative binding to lactose, a galectin-3 agonist, is similar to normal binding at low concentrations. Those results suggest that the structure of galectin-3 undergoes a change after binding to immobilized oligosaccharides, where the immobilization presents a scaffold and then induces oligomerization, resulting in cooperative binding. Importantly, no such positive, cooperative binding is observed for other galectin family members that lack an intrinsically disordered region.
Using surface plasmon resonance (SPR) analysis, Hughes’ group found that, for concentrations at which the abovementioned cooperative binding phenomenon occurs, the dissociation rate of galectin-3 from its ligand oligosaccharides is reduced in the presence of the non-lectin region, thereby stabilizing the binding between galectin-3 and its ligand36. In 2001, they took electron microscopy images of shadowed galectin-3, which showed that most molecules were monomers. In contrast, multimers, like amyloid, were observed for molecules consisting of only the non-lectin region of galectin-3. They therefore speculated that the presence of the CBD suppresses the self-association of non-lectin regions40.
Similar to the self-association of the non-lectin region, reports began to appear around 1998 suggesting that the CBDs of galectin-3 also interact with each other37–39, and that the non-lectin region of galectin-3 interacts loosely with the CBD when its ligand was absent34.
These early studies suggest that CBD–CBD interactions, in addition to the non-lectin region’s self-association, is involved in galectin-3 oligomerization. Furthermore, oligomerization may also be regulated by interactions between non-lectin domains and the CBD.
As discussed below (section 8), LLPS generally occurs when proteins and nucleic acids (RNA) that have very flexible structures reach their “saturation concentration” or “solution limit concentration” in the local area. Thus if galectin-3 causes LLPS, it is necessary that the concentrations of its intrinsically disordered region, CBD, and ligand reach locally saturating concentrations.
The above biochemical studies revealed that the galectin-3 ligand oligosaccharides must be immobilized on the surface and that a certain concentration of galectin is required for positive, cooperative binding. This may be related to the saturation concentration required for LLPS. However, SPR results have suggested cooperative binding of galectin-3 regardless of the amount of glycoprotein immobilized on the surface36. Further investigations are therefore required regarding the local state when this cooperative binding takes place.
Since the 1990s, studies have suggested that galectin-3, which exists as a monomer in the absence of its ligand, induces cell aggregation and cell-to-cell interactions, and also causes cross-linking of membrane proteins to regulate their dynamics. It is therefore relatively easy to imagine that oligomerization, which has been biochemically suggested in vitro, is related to those activities. However, no studies had demonstrated that oligomerization actually occurs on the cell surface at 37°C. We attempted to address this question in 2007, using fluorescence resonance energy transfer (FRET). FRET signals involve fluorescent light emitted when the distance between the substances is very close: i.e., when the distance between proteins A and B is ≤ 10 nm, the fluorescent substance attached to protein B (acceptor: e.g., Alexa 555) is excited at the wavelength emitted from the fluorescent substance attached to protein A (donor: e.g., Alexa 488). Under conditions where only Alexa 488 of protein A is excited, the fluorescence emitted from Alexa 555 is the FRET signal. Using this technique to examine the distance between galectin-3 molecules that bind to the cell surface, it is possible to show that galectin-3 oligomerizes during cell aggregation and lattice formation on the cell surface41.
We used two types of galectin-3-labeled products (Galectin-3-C-Alexa 488 and Galectin-3-C-Alexa 555) in which the C-termini of galectin-3 were modified by either Alexa 488 or 555. These two types of fluorescent galectin-3 co-exist on the cell surface of neutrophils, where neutrophils bind to vascular endothelial cells, and FRET signals were detected. In contrast, when CBDs without non-lectin regions (CBD-C-Alexa 488 and CBD-Alex 555) were used, no FRET signal was detected even if each CBD was present at the same position as confirmed by microscopy. This suggests that, during galectin-3-mediated cell binding (in this case, binding between neutrophils and vascular endothelial cells or between neutrophils), the CBDs of galectin-3 are in close proximity on the cell surface, and this CBD association/interaction requires the presence of the non-lectin region. These results thus suggest that oligomerization of galectin-3, indicated in vitro, also occurs on the cell membrane.
In 2004, Brewer’s group estimated the ratio of galectin-3 to lactosamine units in the precipitate formed by mixing very high concentrations (150 µM) of galectin-3 and galectin glycan ligands in solution. Based on the observed ratio, they speculated that galectin-3 could form at least pentamers42. However, the existence of this type of oligomer with a valence of 5 under physiological conditions remains unclear.
Notably, this report mentions that the oligomers of galectin-3 are assumed to be heterogeneous with no specific structure. This paper also clarifies that oligomers of galectin-3 are caused by the involvement of both the intrinsically disordered region and the CBD, and does not necessarily have to be a pentamer. However, the term “pentamer” has a particular impact, and this pentamer was illustrated resembling a flower with 5 petals (CBD) and 5 pistils (non-lectin region), including in Nature and other review magazines, probably because of its attractive appearance making it easy to understand visually.
However, this illustration may give readers an unnecessarily stereotypical view, suggesting that galectin-3 oligomers are structurally stable and not flexible, like the C-type lectin collectin(Note 4). It should be noted that galectin-3 oligomer is not always a pentamer.
Note 4: Collectins are C-type lectins that consist of a CBD, a collagen-like structure domain, and a neck structure domain with a coiled-coil structure. The trimer forms one unit of collectin, which further multimerizes. The sugar-binding site of the trimer is supported by the neck structure and has a fixed equilateral triangular structure, which is often illustrated as a bouquet. CBDs of collectins themselves have weak affinities for mannose, fucose, and glucose, indicating little sugar-binding specificity for a single sugar-binding site. However, the trimeric, equilateral triangular structure gives the collectin glycan-binding specificity: it does not bind to sugar chains in the host, but binds only to repetitive sugars on microbial membranes56.
As noted above, the oligomerization of galectin-3 and its positive cooperative binding are detectable at physiological concentrations when its ligands are present on the membrane; however, elucidating the precise biochemical state of the oligomers under these conditions is challenging. Other approaches using conditions under which galectin-3 and its ligands are uniformly dispersed may be necessary to obtain more accurate information.
In 2012, Leffler’s group used fluorescence anisotropy to study galectin-3 oligomerization in the presence of the soluble galectin-3 model ligand glycoprotein, asialofetuin43. In the presence of asialofetin 20 µM, more galectin-3 molecules than the number of glycan ligands were involved in the binding at galectin-3 concentrations ≥ 30 µM. Accordingly, Leffler et al. proposed that CBD-to-CBD multimerization contributes to galectin-3 oligomerization, rather than oligomerization of the N-terminal non-lectin region.
Unfortunately, the figures in the published paper cover a wide scale from 0–150 µM, making it difficult to determine from the graphs whether the above phenomena occur at the relatively low concentrations of ~1–2 µM, at which previous studies showed cooperative binding and oligomerization. It is therefore unclear if the interaction between galectin-3 and soluble ligands leads to excessive CBD aggregation, as described above, even at physiological concentrations. When galectin-3 CBD alone, or mutated galectin-3 with reduced sugar binding was used, similar excessive CBD aggregation was observed, albeit at higher concentrations of galectin-3 (> 75–90 µM). Based on these results, Leffler et al. proposed that the oligomerization of galectin-3 by soluble ligands requires some initial aggregation, as a core, followed by oligomerization between CBDs.
In 2014, Guerlesquin's group used dynamic light scattering to investigate if the molecular sizes of monomeric galectin-3 (Note 5) and CBD were altered in the presence of lactose (Galβ1-4Glc) or lacto-N-neotetraose (LNnT: Galβ1-4GlcNAcβ1-3Galβ1-4Glc)44. Thirty-three percent of galectin-3 (when the concentration of 37 µM was used) increased in molecular size from 3.14 nm to 4.64 nm in the presence of a low LNnT concentration (70 µM), while 91% of galectin-3 increased its size to ≥ 100 nm at a higher LNnT concentration (140 µM). In contrast, CBD remained monomeric under the same conditions and the molecular size did not change, and both galectin-3 and CBD remained monomeric in the presence of lactose (8 mM), in contrast to LNnT.
Note 5: Galectin-3 used here was His-tagged. Galectin-3 is usually purified using a lactose column, the bound lectin is extruded with lactose, and the lactose is then removed, so there is little possibility that the purified lectin contains sugar. This study used galectin-3 purified using the His tag. Up to 67% of galectin-3 was reported to be multimeric immediately after His-tag purification. The authors therefore further purified the galectin-3 and then performed oligomerization experiments using only the monomer. Importantly, it was reported that galectin-3 stably remained monomeric after purification to the monomer. Furthermore, because the majority of CBD was monomeric even immediately after His-tag purification, the galectin-3 oligomer seen immediately after purification was not a His-tag-related oligomerization, but rather it is conceivable that some sugars that were not removed in the first His-tag affinity purification (for example, Escherichia coli-derived lipopolysaccharides) triggered the oligomerization.
Similar to the Leffler et al. report, the above experiment used a very high concentration of galectin-3 compared with the extracellular physiological concentration. However, the results suggest that non-lectin region-dependent oligomerization occurs when high affinity ligands are present at high concentrations. Furthermore, when comparing the nuclear magnetic resonance (NMR) spectra of galectin-3 and CBD in the presence of LNnT, several spectral peaks of galectin-3 were reduced, whereas no such phenomenon was observed in the presence of lactose, suggesting that the decrease in this peak was related to galectin-3 oligomerization. Furthermore, as previously predicted40, 45, their new NMR analysis confirmed that the non-lectin region contacts the F- and S-face sugar-binding sites of CBD (Fig. 2A -Galectin-3). These results suggest that galectin-3 binds to high affinity ligands and dislodges the non-lectin region that was previously in contact with the CBD in the absence of its ligand. This dislodged region first induces oligomerization with other non-lectin regions of galectin-3, leading to CBD-to-CBD oligomerization.
A subsequent detailed NMR analysis by Mayo’s group in 2016 suggested that repeats of PGAX (proline-glycine-alanine-any amino acid) within the intrinsically disordered region contacts the F-face of CBD46. Furthermore, Gabius and Romero’s group in 2018 succeeded in crystallizing a CBD with three repeats representing the 7th to 9th repeats of the intrinsically disordered region near the CBD. Similar to the above NMR analysis, the results confirmed that this repeat region contacts the F-face of the CBD47.
Galectin-3 and its CBD are monomeric in solution and the CBD-only crystal is also monomeric48. However, the crystal of the abovementioned truncated galectin-3, which contains 3 repeats of the intrinsically disordered region, occurs as a tetrameric structure. Further studies are needed to elucidate the biochemical significance of this tetramer, which forms a channel-like structure with 4 CBDs of galectin-3, while the analysis again suggests that CBDs interact with each other during the process of oligomerization.
The binding of galectin-3 to immobilized glycolipid GM1 was analyzed by X-ray reflectivity in 202249. According to this report, the CBD of galectin-3 bound to GM1 was 3-4 nm, which was similar to the results of previous analyses37,44. The non-lectin region protruded away from the GM1 immobilized membrane and its estimated length was about 2 nm. The size of GM1-bound galectin-3 inferred from this analysis does not necessarily fit with the expected size of the stacking of galectin-3 oligomers proposed by other studies of its interaction with immobilized glycoproteins. In addition, the non-lectin region, which should be 50 nm37 in the fully stretched state, was only 2 nm.
What does this indicate? Because it is unknown if the binding of galectin-3 to GM1 occurs in a positive cooperative manner like membrane-anchored glycoproteins, it remains unclear if galectin-3 bound to GM1 undergoes oligomerization. Assuming that the non-lectin region is oligomerized in the extended state (50 nm)37, the region is not extended (2 nm) when galectin-3 binds to GM1. Does this suggest that this region is not oligomerized? Although it is only speculation, it is possible that the non-lectin region cannot dissociate from the CBD even if the CBD is bound to GM1 (for example, the state in Fig. 2B-2), which could explain why GM1-binding galectin-3 is small. Further analysis is required to address these questions.
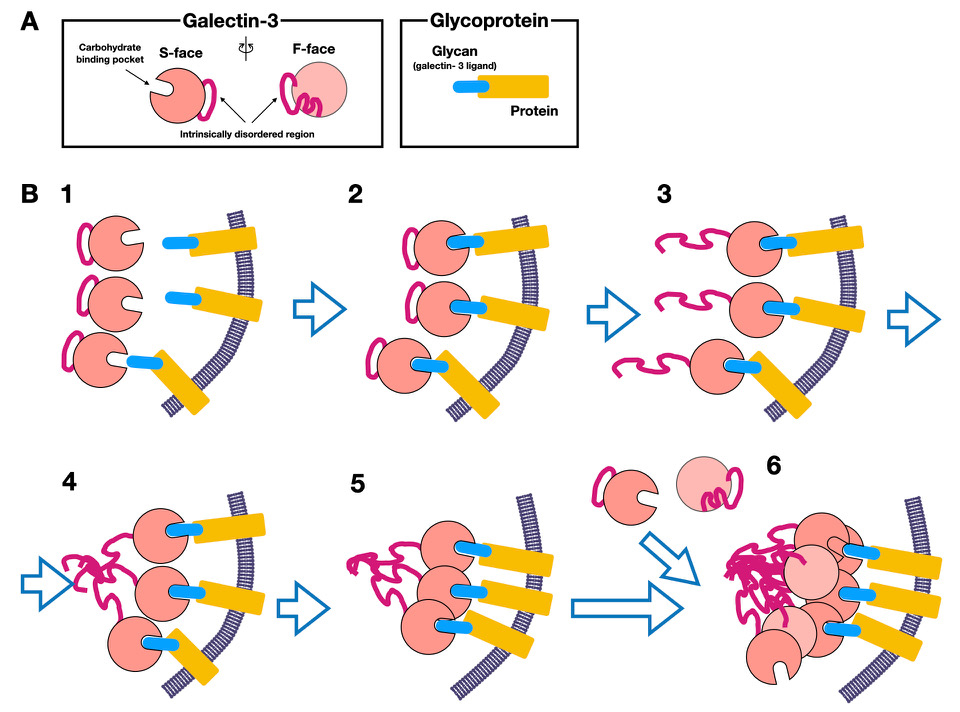
Because recent research on galectin-3 oligomerization has focused on elucidating the molecular mechanism, the analytical conditions have included high concentrations of galectin-3 and water-soluble ligands, instead of using physiological conditions in which lower concentrations of galectin-3 are added to sugar chains immobilized on membranes. Not all the results are necessarily consistent; however, the points of consensus between studies using immobilized ligands conducted before 2000 and recent analyses indicate that the process of oligomerization of galectin-3 can be summarized as follows (Fig. 2B).
1) Galectin-3 binds to specific ligands (e.g., glycoproteins) on the membrane (Fig. 2B-1, 2), resulting in the release of the non-lectin region (particularly intrinsically disordered regions), which is loosely bound to the F-face of CBD (Fig. 2B-3).
2) The intrinsically disordered region that is released from the CBD first oligomerizes (Fig. 2B-4), followed by oligomerization of the adjacent CBDs, resulting in a relatively small level of oligomerization (nucleation aggregates) accumulating on the membrane surface (Fig. 2B-5).
3) The oligomerization (or clustering) of CBDs then further promotes the oligomerization of galectin-3 (Fig. 2B-6).
4) This phenomenon leads to positive cooperative binding, which stabilizes the binding with the glycan ligand.
However, I would like to further consider if the clustering of CBD that occurs during the initial (nuclear) aggregation (Fig. 2B-3 to 5) and further oligomerization (Fig. 2B-6) occur via the same molecular mechanism. The first phenomenon presumably involves subtle conformational changes in CBD induced by its ligand binding. Most biochemical results indicate that the interaction or aggregation of the CBD alone without the non-lectin region is rare under normal conditions, suggesting that the affinity between CBDs is rather weak. This would suggest that the initial CBD clustering requires intimate proximity between the CBDs, which is only possible when oligomerization-like binding (LLPS-induced?) of the intrinsically disordered regions first occurs. In addition, the subsequent participation of CBDs in oligomerization by attraction to the aggregation nucleus may also be somehow related to the intrinsically disordered region. This oligomerization process, which has many unexplained points, is a phenomenon unique to galectin-3 and may be related to LLPS. Finally, I would thus like to outline this LLPS phenomenon, to provide a bridge to the second part of this glycoforum to be published later.
Biological LLPS (Note 6) is a state in which several biomacromolecules (proteins, RNA, and possibly glycans) are brought together by weak interactions. These molecules become more stable when separated into two phases than when mixed homogeneously, in a phenomenon known as “phase” separation. For example, salad dressing may be a familiar visible example of LLPS: the dressing contains seasonings and finely chopped vegetables as additives, in addition to oil and vinegar. These additives separate into an oil phase and a vinegar phase according to their respective characteristics. However, even if it is well-mixed, small aqueous droplets are formed after a while and eventually become large droplets. As shown by this example, when LLPS occurs, droplets are often formed because the separation into two phases is more stable. These droplets are stabilized by multiple interactions such as static electricity, cation-π, and π-π, and contain many intrinsically disordered proteins and multivalent ions such as RNA. Because droplets are formed through many different weak interactions, they are mobile and can form and dissolve rapidly in response to subtle changes in temperature, pH, and concentrations of salt and small molecules (e.g., ATP). Notably, this process may result in some biomolecules being highly concentrated in the droplets and certain substances being excluded from the droplets. It can thus be easily imagined that, for example, specific reactions proceed efficiently in the droplets as a result of the concentration and elimination of molecules caused by this LLPS phenomenon.
Note 6: Please refer to Kentaro Shiraki's book “Phase Separation Biology” [Tokyo Kagaku Doujin Publishing (in Japanese)] for a more detailed theory of LLPS and LLPS-related biological phenomena. This book covers aspects from fundamental biochemical issues to applications. Unfortunately, there is currently no English translation; however, reviews of LLPS in various fields have been written in various scientific journals in recent years, so please refer to these for details.
LLPS requires the presence of intrinsically disordered proteins43. Proteins (for example, many enzymes and lectins) generally have specific activities and functions as the nascent polypeptides that emerge from the ribosomes are folded to form tertiary or higher-order structures. However, some proteins contain regions with no specific structure (no tertiary structure), and are referred to intrinsically disordered proteins. Notably, only 4.2% of proteins in eubacteria carry intrinsically disordered peptide regions consisting of 30 or more amino acid residues, compared with 33% in eukaryotic cells44. The increase in intrinsically disordered proteins with eukaryotic evolution may reflect the increasing efficiency and refinement required by more complex systems in eukaryotic cells. Indeed, many proteins involved in transcription/translation and signal transduction, such as transcription factors, kinases, nucleic acid-binding proteins, and RNA polymerases, are intrinsically disordered proteins.
Galectin-3 is the only member of the galectin family that has an intrinsically disordered region, and may be the only mammalian lectin with such a long disordered region. In 2015, Dennis proposed that “galectin-3 induces LLPS on the cell membrane through its oligomerization and forms microdomains”, and that this phenomenon comprised the cell surface galectin lattice, as previously suggested 2. In 2017, Huang’s group in Taiwan showed for the first time that oligomerization of galectin-3 does indeed induce LLPS in vitro3. Although only three papers, including those by Huang’s group, have reported on galectin-3-induced LLPS3, 5, 6, their suggestive findings are likely to be related to both the process of oligomerization and the wide range of functions of galectin-3 both inside and outside the cell.
Quoting from Dr. Shiraki's book52 about LLPS: “LLPS biology is not the chemistry of matter in terms of molecules and structures, but rather the chemistry of phenomena in terms of states and interactions. Given the answer that it is due to biological phase separation, the questions left unsolved are listed as follows. Why do hundreds or thousands of complex metabolisms proceed without melee? ---Why do certain reactions proceed efficiently in a state where various molecules are present at high concentrations? Why are there so many intrinsically disordered proteins that are supposed to have no structure and no function? Why does signal transduction flow in such a way that information appears to gather once?”
LLPS is expected to cause paradigm shifts in various biological fields in the future. Indeed, LLPS has been selected as one of Science’s 2018 Sciences of the Year. However, it is necessary to bear in mind that most research related to LLPS utilizes in vitro experiments with physiologically very high concentrations (e.g., mg/ml), or cell conditions under which proteins are forcefully expressed at high concentrations. Given that LLPS research was initially developed in the field of physicochemistry, experimental techniques for applying it to cell biology are still under development and are currently not necessarily optimal.
Based on his own research experience, Robert Tjian, a leading scientist in the field of transcriptional regulation, warned that one should not jump lightly to the conclusion that an observed phenomenon is related to LLPS, given that we have not yet developed or optimized technologies to show if LLPS actually occurs under physiological conditions53. He warned that it would be better to put aside LLPS, and rather use the concept of "hubs", until suitable technologies can determine if the state of aggregated biomacromolecules observed inside cells is indeed fluid droplets.
Many scientists are understandably cautious when considering LLPS. However, what if the biological reactions and processes described above (e.g., signal transduction, transcription, and other events occurring in the non-membrane space) are actually LLPS-related? Most experimental systems used to date have difficulty reliably detecting weak and otherwise ambiguous interactions involving a variety of macromolecules, leading to the possibility that we may have missed many biologically important processes involving LLPS. Different or misleading conclusions may also have been drawn because LLPS rapidly changes the degree of molecular aggregation due to subtle differences in factors such as temperature, salt concentration, and pH.
For example, the study of molecular interaction networks commonly uses biochemical methods of immunoprecipitation and affinity-based purification involving cell disruption and dilution at low-temperature. In many cases, detergents are also added “to prevent nonspecific interactions”. The aggregation/clustering of proteins and molecules, which relies on weak interactions (the cornerstone of LLPS), would therefore be missed under these conditions. Alternatively, because intracellular components are mixed during the experimental processes, it is also possible that binding between proteins, which does not occur inside cells, is also picked up. Furthermore, it remains unknown whether molecular interactions in LLPS can be detected by proximity-dependent labeling47, which has recently been used to study interactions in the native state inside cells.
The interactome database has been accumulated based on the reports of millions of protein interactions using classical approaches, and systems biology analysis has been developed based on this database. However, how accurate would the analysis be if the abovementioned concerns were raised? Interactome information may thus need to be interpreted cautiously in the future, with the possibility that “facts” based on the experimental results accumulated to date may change significantly. Thus although it is important to proceed with research carefully, without being influenced by the popular trend of LLPS, we also need to review the classical view of molecular interactions with skepticism.
Notably, research into the role of extracellular galectin-3 in LLPS may proceed slightly faster than that for LLPS driven by other molecules, because it is possible to conduct research in which physiological amounts of galectin-3 can be added to the extracellular space. Thus, it may soon be possible to clarify if the galectin lattice, as the “hub” that binds to glycoproteins present on the cell membrane, is LLPS. Similarly, the relationship between LLPS and autophagy, which is induced by the recognition of damaged phagosomes and lysosomes by galectins, may also be elucidated relatively soon.
In the second part of this forum, I will discuss how the LLPS phenomenon is induced by galectin-3 and how it is related to various intracellular and extracellular functions of galectin-3.
I would like to thank Dr. Jun Iwaki for his critical reading and Dr. Jun Hirabayasi for his constructive advice and thorough edition.