
Jun Hirabayashi
Tokai National Higher Education and Research System, Nagoya University, Japan. Ph.D, Science.
After graduated from Tohoku University (Master of Science), he started his professional carrier at Teikyo University under supervision of Prof. Kenichi Kasai for the investigation of animal lectins. On the occasion of GlycoXV (Tokyo, 1999), he proposed the concept glycome; for this realization, he moved to National Institute of Advanced Industrial Science and Technology (AIST, Tsukuba) in 2002, and was involved in a series of national projects for glycan engineering, while he was a deputy director in Research Center for Medical Glycoscience (2006~), prime senior researcher of Research Center for Stem Cell Engineering (2012~). Now, he is a designated professor in Institute for Glyco-core Research (iGCORE), Tokai National Higher Education and Research System, Nagoya University, as a project manager (HGP), while being a vice president of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology. (JCGG). He is also a visiting professor of Kagawa University (2003~) and Yokohama City University (2019~).

Ryuichiro Suzuki
Associate professor at Akita Prefectural University since 2022. He studied the rational engineering of xylanase under supervision of Prof. Tsunemi Hasegawa and Dr. Atsushi Kuno in the Graduate School of Science and Engineering, Yamagata University. After graduating from Yamagata University (Ph.D, Science) in 2005, he studied the structure and function of R-type lectins under supervision of Dr. Jun Hirabayashi at the National Institute of Advanced Industrial Science and Technology. In 2006, he moved to the laboratory of Prof. Shinya Fushinobu (University of Tokyo) and studied the structure and function of enzymes involved in the metabolism of milk oligosaccharides (2006–2010). He then moved to the laboratory of Dr. Kazumi Funane at the National Food Research Institute, where he studied the structure-based design of cyclic isomaltooligosaccharides metabolism-related enzymes (2010–2012). He changed to the status of assistant professor in 2012 at the laboratory of Prof. Eiji Suzuki in Akita Prefectural University. At present he is associate professor at the same laboratory (2022–). His research interests are cyanobacterial α-glucan metabolism and structure-function relationships of the related enzymes.
Galectins are characterized as a group of evolutionarily related proteins having a conserved unique sequence with the ability to bind galactose (rigorously to bind β-galactoside). To date, their diverse biological distributions and functions have been described; however, the origin of galectin has only scarcely been discussed. Curiously, all of them so far investigated lack a signal sequence required for secretion, while they are assumed to function in extracellular spaces, where a vast amount of galectin-binding glycans exist. In fact, regardless of biological species, all the galectins are biosynthesized as “cytosolic proteins”. This suggests that they have intrinsic functions in the cytosol. In this chapter (Part 1), we describe the origin of galectins in light of their cytosolic features. In the following chapter (Part 2), We attempt to discuss the most fundamental issues of galactose, to which all galectins bind, by referring to the origins of monosaccharides and their chirality.
As has been described repeatedly in this series and elsewhere, galectins bind to a structural unit of disaccharides like N-acetyllactosamine (LacNAc) 1-3. This corresponds to the 4th of the seven wonders of galectin described by Kasai4 i.e., why are all galectins galactoside-specific? Let’s first examine this from a structural viewpoint.
There are many lectins that recognize galactose, including galectins5. All of them commonly require recognition of an OH group at the 4th position of galactose (C4-OH) with which multiple hydrogen bonds form. Fig. 1 shows steric structures of representative galactose-binding lectins derived from different origins: they are galectin (Pfam ID: PF00337), Erythrina (Indian coral tree) lectin (PF00139), C-type lectin (PF00059), ricin B chain (PF00652), Pseudomonas lectin PL-IL (PF00059), jacalin (PF01419), Toxoplasma lectin TgMIC4 (PF00024), and Escherichia coli enterotoxin (PF01375). In all interactions of galactose-binding lectins with galactose and its derivatives, hydrogen bonding to C4-OH is a key element. Hydrogen bonds are formed if a hydrogen atom (proton) is placed between two hetero atoms (e.g., N and O) linearly, within an appropriate distance (2.5–3.5 Å). Although the binding energy of each hydrogen bond is relatively weak (10–40 kJ/mol) compared to the energy of a covalent bond and coordination bond, the addition of a single hydrogen bond increases the binding affinity approximately one order of magnitude Note 1).
Note 1) When discussing the binding mechanism between lectins and carbohydrates, hydrogen bonding is important as well as Van der Waals interaction (including “stacking” by aromatic amino acid residues) and hydrophobic interaction; however, the hydrogen bond is unique in that it rigorously defines both the distance and angle to make solid interaction with a binding partner, and thus, is most critical for acquisition of high selectivity for carbohydrate ligands.
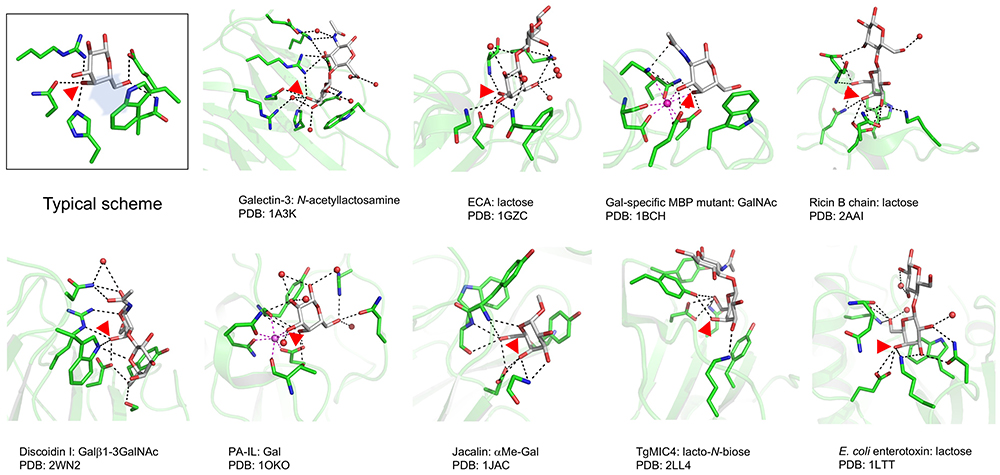
Except for this common property, however, the recognition patterns of the second and third hydrogen bonds are diverse. Namely, galectins require C4-OH and C6-OH (a property that is evolutionarily conserved), while many other lectins require C3-OH and C4-OH, and galectin-like recognition requiring C4-OH and C6-OH is rather rare6. Another common feature of galactose-binding lectins is stacking interaction between the hydrophobic face, also called the B-face Note 2) of the pyranose ring and aromatic residues, which include tryptophan, tyrosine, and phenylalanine7. In the case of galectins other than frog galectin8, this role is played by an evolutionarily conserved tryptophan (Trp68 in human galectin-1).
Note 2) The numbering of carbon atoms in the ring is clockwise at the A-face of the sugar and counterclockwise at the B-face 9.
As described, galactose-binding lectins including galectin are attracted to the C4-OH group of galactose. Of note, here, this C4-OH group is maintained in the axial configuration relative to the pyranose ring (Fig. 2), when the galactose molecule exists in a stable chair form termed 4C1 Note 3). Indeed, the unique feature of an axial OH group at the C4 position of galactose has many admirable features as a recognition saccharide10.
Note 3) Like cyclohexane, the pyranose ring adopts two possible low energy conformations that look like a ‘chair’ (C), termed 1C4 and 4C1, where the numbers indicate the carbon atoms located above or below the reference plane formed by the C2-C3-C5-O square.
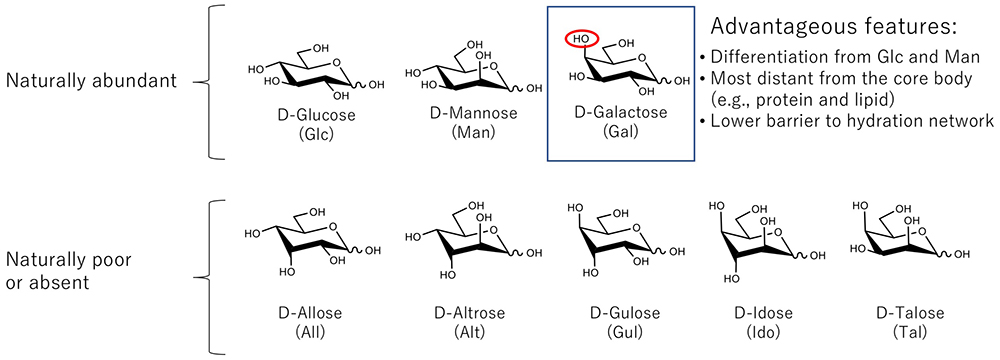
First, with the axial OH group at position 4 being farthest from the reducing terminal, galactose may be more easily recognized. Second, the axial C4-OH group can be a good “marker” to distinguish it from other major monosaccharides like glucose and mannose. Third, the presence of the axial OH group is considered to contribute to lowering the physical barrier created by the hydration network which surrounds each monosaccharide. It is known that sugar hydration is affected by the number of equatorial OH groups interacting in a manner which forms a long-lived hydration structure11,12. All of these properties are advantageous to the function of galactose over other common aldohexoses (glucose and mannose) as a recognition saccharide. Galactose is located at the non-reducing terminal position presumably because galactose appeared at a later stage of evolution compared to glucose and mannose as hypothesized by one of the authors (JH). This point is described in the next chapter.
It is an open question when galectin developed as a molecule with β-galactoside-binding ability and the ability to function in diverse biological phenomena. Of note, galectins are widely found in metazoans, but not in bacteria and plants13-15. Thus, it is estimated that galectins did not originate soon after the birth of life (ca. 3.8 billion years before), but probably sometime after the birth of eukaryotes, which evolved from prokaryotes.
The same is likely true for another representative animal lectin family, C-type lectins16. Indeed, genes encoding many members of these lectin families are easily found in the genome of the nematode Caenorhabditis elegans and at least a few of the candidate genes proved to be functional17-19. On the other hand, galectins but not C-type lectins have been found in sponges20. In addition, galectins have been extensively found in mushrooms, while no galectin has been identified in yeasts. Anyway, it is not conceivable that these lectins originated in ancestral bacteria, and a more likely possibility is that they achieved their present status as a result of a long trial-and error “adaptive radiation” process of body plan evolution toward metazoans.
Namely, it is assumed that galectins were naturally selected as "carbohydrate-recognition molecules" at the multicellularity stage of evolution: they were selected to regulate complex cell ‘sorting’ mechanisms for development, or to build up systems with both symbiotic and defensive functions ranging from innate to acquired immunity. Probably, they were involved in interacting with various exogenous microorganisms, through which their role in metazoans gradually expanded21-23. However, it is still an assumption that such an event really happened under adaptive radiation circumstances. Here, we’d like to remind you of the mysterious fact that “galectins lack a signal sequence required for secretion”, which is the second of the seven wonders of galectins described by Kasai: in other words, “Why are they extracellularly located in spite of the fact that they were designed to be intracellular proteins?”4.
All galectins retain inherent properties of cytosolic proteins, with no exception. That is, 1) they don’t have a hydrophobic signal sequence for secretion. 2) They are not translocated to the endoplasmic reticulum and Golgi apparatus. 3) Even if they are equipped with a glycosylation-attachment site (N-X-S/T), they are not subjected to N-glycan modification. 4) Cysteine residues exist as free thiol and do not form disulfide bridges (at least in the cytosol), and a few galectin members lose their lectin activity under oxidative conditions. Further, 5) N-terminal amino acid is acetylated in the cytosol. These properties are quite contrasting to those of C-type lectins representing another family of animal lectins (Table 1).
| Properties | Galectins | C-type lectins |
|---|---|---|
| N-terminal hydrophobic signal | No | Yes |
| Glycosylation (N-glycan) | No | YesNote 4) |
| Phosphorylation (Ser/Thr) | Yes | No (extracellular domain) |
| Disulfide bridge | NoNote 5) | Yes (evolutionarily conserved) |
| N-terminal acetylation | YesNote 6) | No |
| Metal requirement | No | Yes (coordination bond to Ca2+) |
| Carbohydrate specificity | βGal | Gal, Man, Fuc, etc. |
| Tandem-repeat type | Yes | Yes |
Note 4) There are a substantial number of potential glycosylation sites in amino acid sequences in C-type lectins; however, there are relatively few cases for which actual glycosylation has been confirmed.
Note 5) Galectin-1 of human and other mammals have 6 cysteine residues, which should be maintained for activity in the free state by the presence of an appropriate thiol-reducing reagent like 2-mercaptoethanol. Once this lectin is placed under oxidative conditions, some cysteine residues undergo significant structural change possibly induced by disulfide bond formation, and hence, sugar-binding activity is lost.
Note 6) In eukaryotes, galectins are subjected to N-acetylation, whenever possible, because the responsible enzyme is located in cytoplasm; however, the specificity largely depends on the N-terminal amino acid. The first demonstration of this modification was made in chicken 14K galectin24, while in the case of chicken 16K galectin, the initial methionine is not removed, and thus, N-acetylated25. This is attributed to inherent specificities of the two relevant enzymes, methionine aminopeptidase and N-acetyltransferase26. Mass spectrometry and chemical modification analyses can determine this post-translational modification, while nowadays this is rarely done.
When the first complete cDNA for chicken 14K galectin was cloned27, it was pointed out that chicken 14K galectin lacks a signal sequence for secretion, albeit the protein is obviously secreted. Since then, 36 years have passed, and substantial progress has been made with regard to novel functions associated with autophagy and lysosomal degradation events in the cytoplasm28-30. Also, it became evident that not only galectins but also some other exceptional proteins lacking a signal sequence, such as interleukin-131 and basic fibroblast growth factor, are secreted32. This implies that there is still a revealed principle to be expanded33,34.
On the other hand, being synthesized as cytosolic proteins may be a key to solving another question; i.e., about the origin of galectin. In this regard, one might speculate that the primordial galectin was a cytoplasmic scaffold/aggregate-forming protein with broader specificity, and subsequently acquired affinity for β-galactosides, followed by positive selection and gene duplication (Jim Dennis, personal communication; also see refs. 35 and 36). This results in diverged functions of individual galectins, the evolution of which may or may not have been completed yet (i.e., evolutionary change is ongoing15). For instance, other than binding to the conventional ligand N-acetyllactosamine, the C-terminal carbohydrate-recognition domain of galectin-8 also binds a 9 amino-acid sequence of NDP52, to recruit ubiquitin receptors, such as NAP1 and TANK, which leads to autophagy and suppression of bacterial infection (for more detail, see ref. 37).
When considering the evolution and these present-day features of galectins, we have come across another fundamental question, i.e., “When was galactose born?”, because without galactose the present-day galectin a priori should never have developed. We will discuss this issue in the next chapter.
Authors thank Dr. Jun Iwaki (Tokyo Chemical Industry Co. Ltd., Tokyo), who contributed to the preparation of figures and joined the discussion. The authors also thank Dr. Jim Dennis (Mount Sinai Hospital, Toronto), who helped discuss the origin of galectins. Dr. Sachiko Sato (Laval University, Quebec City) is also acknowledged for her continuous commitment to the editing.
Authors sincerely dedicate this manuscript to the memory of Prof. Hans-Joachim Gabius, who made great contributions to galectins research through addressing many unsolved questions including those of galectins evolution and functions.