
Hakon Leffler
Hakon Leffler, received MD 1974 and PhD 1981 from Göteborg University, Sweden, 1985-86 joined group of SH Barondes at UC San Diego and moved with him to UC San Francisco 1986-1997, since then at Lund University, Sweden, now as Senior Professor. PhD and early research included isolation and structure of glycosphingolipids and their role as receptors for bacterial adhesion. From 1985 main research has been on galectins, including biochemical (discovery, specificity), structural and cellular aspects of galectins. In collaboration initiated development of potent small molecule inhibitors, some of which are now in clinical trials against fibrotic disease and cancer. For this co-founder of company Galecto Biotech AB, now Galecto Inc. (NASDAQ: GLTO).
The natural ligands of galectins are mainly glycoproteins and to some extent possibly glycosphingolipids. The binding affinity and specificity of galectins for small free saccharides has been studied extensively, but not the binding of saccharides when attached to natural glycoproteins or glycolipids. Galectins often bind a glycoprotein or cell surface with higher apparent affinity than the corresponding glycans when tested as free oligosaccharides. Here I will argue and provide evidence that this may not be due mainly to multivalent interactions, but instead that extended binding sites on the galectin-carbohydrate recognition domain (CRD) providing higher monovalent affinity makes important contributions. Such monovalent interactions can also be highly selective, where the selectivity comes both from the structure of the glycan bound, and protein parts near where it is attached. Multivalency is instead critical for galectins ability to cross-link ligands in various ways, a key part of their function. The cross-linking depends on the monovalent selectivity, but can also add an additional layer of selectivity and contribute to affinity. The main focus will be on galectins-1 and -3, but other galectins are also included. The equally interesting interaction of galectins with non-glycosylated protein ligands intracellularly is not covered here.
Binding between molecules means that they stay long enough together for the complex to be experimentally observed. Strength of binding means roughly how well the complex resists falling apart when the free components are washed away or diluted.
Binding affinity, measured as association constant (Ka) or dissociation constant (Kd), is defined by the concentration of the complex relative to the free components at equilibrium. In a 1 to 1 interaction, most common here, Ka = [AB]/[A]*[B] and Kd = [A]*[B]/[AB].
Ka is higher with stronger binding, so intuitively attractive, but is measured as molarity-1 which is less intuitive. So we prefer to use Kd, albeit lower with stronger binding, but it is measured in molarity and relates more easily to expected binding of a component with known concentration, e.g. in biological settings. Ka and Kd can be defined also for complexes with more than 1 of either component, as long as a strict mathematical model of the interaction can be assumed. However, in most such more complicated cases in biology this is not possible, and then avidity is instead used to describe binding strength. Avidity is not any extra force per se, but can simply be seen as a measure of binding strength that cannot be easily expressed mathematically.
Binding is determined by a negative difference in Gibbs free energy between the bound complex and the free moieties. This is typically a small difference between two large sums that involve many components, including both enthalpies and entropies, and calculating it is still an unsolved and active research question1-3. Enthalpy change is related to various direct interactions, and is measured, for example, as heat release by ITC. Entropy change is related to statistical number of alternatives, measured, for example, as altered disorder and mobilities of both protein, ligand and associated water molecules. Despite this complexity, the following simplistic consideration is useful4,5. When two molecules form a complex, there is always an entropy penalty because one particle (the complex) has less disorder and mobility as a whole (translational and rotational) than the two separate parts. The magnitude of this loss was estimated to be 15–20 kJ/mol, i.e. around 3 orders of magnitude in affinity at 298 K5. This so called rigid entropy penalty needs to be overcome by the sum of other energies of the interaction (both enthalpies and entropies) for any binding to be observed. But once overcome, adding additional even small interactions, e.g. by adding covalent fragments to the ligand, can have profound effects on enhancing binding. For galectins, one can argue that the binding of galactose in the well-coordinated conserved site is what “pays” for the rigid entropy loss giving Kd values in the mM range (Fig. 1)6 Then adding a glucose residue as in lactose, providing only one -OH forming additional hydrogen bonds, with favorable energy in the 10 kJ/mole range can enhance affinity 100 fold, despite the fact that binding of glucose by itself is too weak to be detected. Adding small artificial moieties on either side of the galactose could enhance affinity by up to 1 million fold7. The added interaction site can be a distance away from the initial site, as likely in galectin glycoprotein interaction, where small interactions in other parts of a large N-glycan and even protein-protein interaction can give important contributions. Multivalency can enhance affinity in a similar way, where first bound site can be said to be paying for the rigid entropy and additional interacting sites act as enhancing fragments.
In all the cases described above, a gain in affinity may be counteracted by any losses due to alteration of the initial binding interaction, interference by linkers, and less than perfect additional interactions. The spacing of Gal-residues of a triantennary N-glycan matching the positions of binding site in a trimeric C-type lectin (the hepatic asialo-glycoprotein receptor) gave 1,000,000 fold enhanced affinity compared to Gal in a monoantennary glycan8, and inspired the concept the glycosidic cluster effect9. However, for galectins, such a strong affinity or avidity enhancement due to multivalency has not been found. For semantic clarity we reserve the term domain for a folded unit of a protein (~130 amino acids), and multivalency for a protein having more than one folded domain. We use the term site or subsite for specific places within a domain where ligands bind. Multiple subsites within one carbohydrate recognition domain, could also have been called a kind of multivalency, but here we avoid the term in this case for semantic clarity.
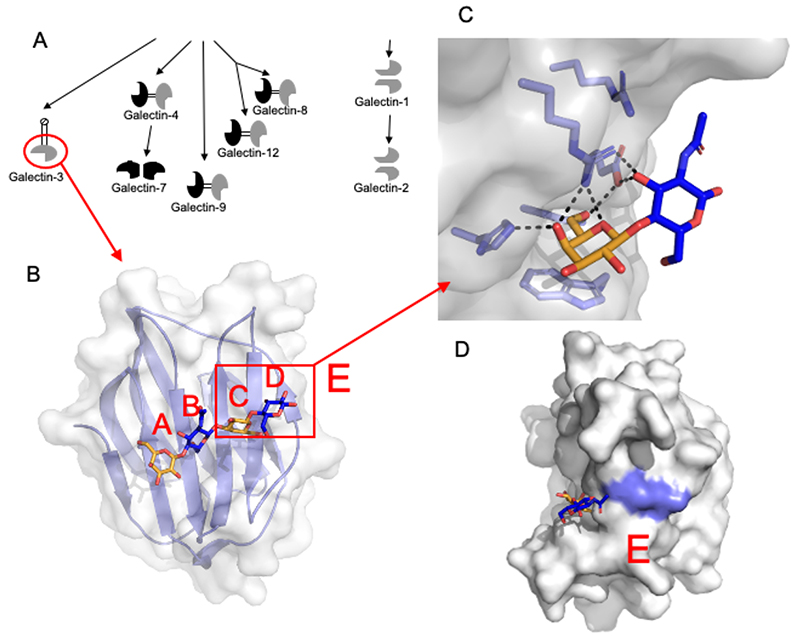
Galectins are defined by one or more about 130 amino acid carbohydrate recognition domains (CRDs), forming a slightly bent β-sandwich and containing a carbohydrate-binding grove on one side, about the length of a tetra-saccharide (Fig. 1A and B). As points of reference subsites in the grove have been named A, B, C and D10, where subsite C is built by a conserved amino acid sequence motif, which coordinates a galactose residue (Fig. 1C), and can be said to pay for the rigid entropy loss on binding, in the way described above. In summary, the binding profiles of galectins to small saccharides predicted a binding site11,12, confirmed by X-ray crystallography, that permits formulation of simple rules of what is likely to bind or not as described below6,13-18.
Monosaccharides in subsite A and B interact less with the galectin than the galactose in site C, but can still strongly enhance or decrease, even prevent binding. Saccharides here include sialic acid, which if linked to 6-position of the Gal, will prevent binding, but if linked to 3-position can enhance it, be tolerated, or prevent it, depending on which galectin. Other saccharides here are α-linked Gal or GalNAc to 3-position of the core Gal (as e.g. part of blood-group determinants). Again these can either enhance or decrease binding depending on which galectin. Finally, GlcNAcβ can be found in subsite B and be extended by another Gal into subsite A giving e.g. galectin-3 the ability to bind an internal LacNAc residue within a poly-N-acetyllactosamine chain.
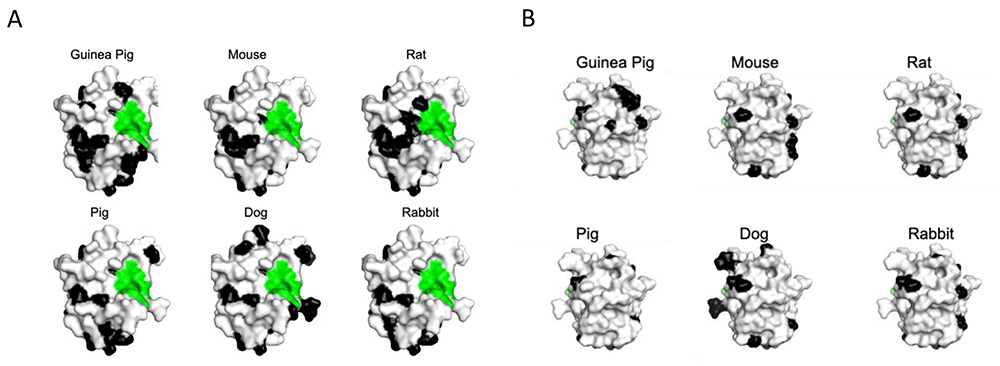
Interestingly, the amino acid sequences building subsite A and B are the least conserved when vertebrate galectin CRDs are compared, both globally and for a specific galectin (Fig. 2). One may ask if this is an adaption to tolerate variations of terminal glycan parts driven by other factors, or an adaption to recognize specific glycan terminal parts more relevant for one species than another. It is also possible that different specificity of sites A-B of related galectins with different tissue expression, reflect adaptation to glycans in the tissue. For example, galectin-2, an intestinal relative of galectin-1 has preference for blood group determinants14, as expressed in intestinal epithelium19, and not 2-3 sialylated glycans, in contrast to galectin-1.
Subsite D also varies. For galectin-1 and 3 Galβ1-4GlcNAc was early found to be the preferred disaccharide in subsite C-D, and often quoted as the main disaccharide binding site for galectins on glycoproteins and on cell surfaces. However, this is not true for other galectins16,20. Galectin-8N, for example, binds Galβ1-3GlcNAc, Galβ1-3GalNAc and Galβ1-4Glc better than Galβ1-4GlcNAc6. Fucosylation of the hydrogen-bond engaged OH of GlcNAc in site D prevents binding11,12. If the ring of the saccharide in subsite D is opened by e.g. labeling by reductive amination13,16, it will no longer represent the native ring closed structure, and usually binds weaker.
A more loosely defined subsite E was included to indicate possibility of further interaction by additional saccharides or aglycones at the reducing side of the saccharide in subsite D. In evaluating experimental binding data, attention to common modification of the reducing end saccharide in site D and added linkers in site E is particularly important. For example, addition of a linked fluorescein moiety as used in direct binding analyzed by fluorescence anisotropy will often enhance binding significantly compared to the underivatized saccharide6,15. Immobilization of a glycan on a surface, as in arrays, will also by necessity add moieties potentially interacting in site E21.
Complex (galactose containing) N-glycans are major binding sites for galectins, since their ablation, e.g. by depletion of the key enzyme Mgat1, results in decreased binding of galectins to a cell surface, almost to zero22,23. Within N-glycans, galectins are most likely to bind a Gal-containing disaccharide in subsite C-D, most often LacNAc. The binding is expected to be affected by modification of this disaccharide in subsite A-B and D, like small saccharides described above, although this has been studied only to a limited extent mainly regarding sialylation, as mentioned in the previous section21,23-25.
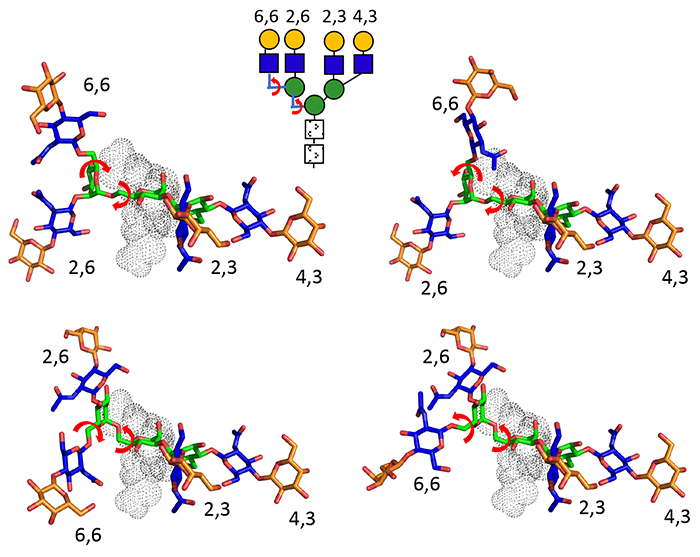
There is also clear evidence that galectin affinity and selectivity vary depending on the number and position of antenna of the N-glycan. In this context, it is worth noting that N- glycans have clearly preferred conformations, despite often being described as flexible26-28. The main rotatable bonds are for the 6 linked Man and the 6-linked GlcNAc for a third antenna, whereas the remainder of the glycosidic bonds are not very rotatable (Fig. 3) because of the exoanomeric effect. For a complex N-glycan, then, one could define a few rather narrow energy valleys defining each preferred conformation26. The depth of these valleys was up to 18 kJ/mol indicating penalties up 1000 fold affecting binding that could occur if the glycan was forced far out of its preferred conformation.
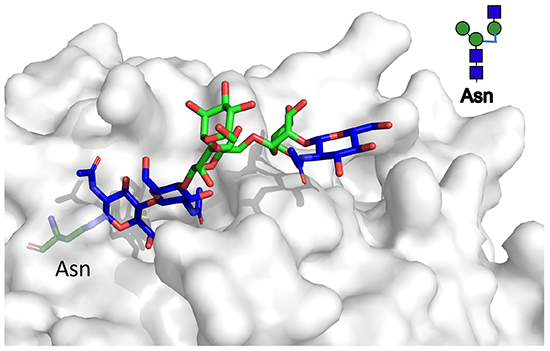
Beyond interaction with the N-glycan itself, binding is also affected by to what the N- glycan is attached to, including which specific position within a glycoprotein. These interactions most likely reflect interaction in the broadly defined subsite E (Fig. 1D). Fig. 4A shows molecular models of galectin- 3 CRD with different non-sialylated common N-glycans, and with a linker fluorescein at the reducing end, as a marker of moieties possibly linked there. All N-glycans have a LacNAc in site C-D, but depending on its position the rest of the N-glycan, and especially the reducing end, points in very different directions, from the point of view of the galectin. This can give different enhancing or hindering interactions with the moieties at the reducing end, and also the part of the N-glycan beyond the LacNAc in site C- D.
Direct evidence supporting (but not proving) the present model comes from fluorescence anisotropy (FA) (Fig. 4B)23. When one of the fluorescein tagged N-glycans was titered with increasing concentration of galectin-3, FA will increase as binding occurs. The concentration with about half maximum increase will reflect affinity (Kd), but also interesting here is the maximum value approached (Amax). This will reflect the mobility of the fluorescein moiety within the galectin-N-glycan complex. Galectin-3 gave the highest Amax value with tri- and tetra-antennary N-glycans having a third antenna 4-linked to the tri-mannose core (the 2,2,4 triantennary in Fig. 4A and the tetraantennary in Fig. 4B), and gave lower Amax values with triantennary N-glycan with the third antenna 6-linked to the trimannose core (2,2,6 in Fig. 4A) and with the biantennary N-glycan23. For the former, the fluorescein moiety and linker comes near the galectin protein surface as shown for a triantennary (top left model in Fig. 4A) and tetraantennary N-glycan (Fig. 4B, top left), but not for the others, suggesting that this proximity hinders motion of the fluorescein. In a model of galectin-1 with the tetraantennary N-glycan (Fig. 4B bottom left) the fluorescein moiety is not near the protein, and indeed the Amax value with galectin-1 is low for all the N-glycans.
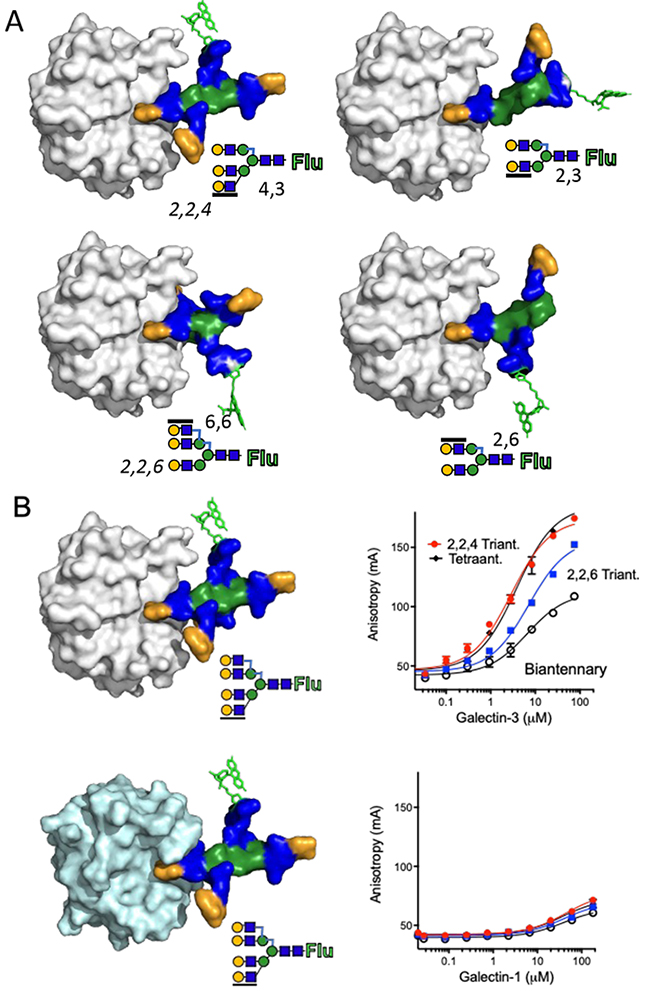
The binding preference with the FA assay agrees with binding of galectin-3 to an array, where there was an even more clear preference for the 2,2,4 triantennary and the tetraantennary N-glycans21. In contrast, the different kinds on N-glycans were found to bind equally well to galectin-3 when tested as soluble inhibitors without addition at the reducing end (in fact having one GlcNAc less there)29. This suggests that a main source of selectivity between different N-glycans comes from how and to what they are linked at the reducing end.
Additional evidence from the glycoprotein point of view is the fact that galectin-1 or galectin-3 binding required the presence of at least tri-antennary N-glycans to bind serum haptoglobin30 and transferrin25, respectively, with functional consequences. In another example, the precise glycosylation site within the glycoprotein CD98 appeared to determine which effect binding of galectin-3 would have, either promoting or inhibiting endocytosis18,31.
Evidence for a role of site E in the galectin also comes from mutation of a residue there (R186S in galectin-315,32 and R74S in galectin-1 (unpublished)) that will essentially abolish binding to glycoproteins, but not binding to the LacNAc disaccharide in site C-D.
Linear polylactosamines (Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAc-R also named i antigens) may be attached to N-glycans, and preferentially to the 6-linked antenna in some cases33. Galectin-3 may bind internal LacNAc residues of these with enhanced affinity11,14. Loss of binding after enzymatic removal of polylactosamines has indicated them be required for some galectin-binding, e.g. to laminin and cell surface in some cases14,34,35. Of particular interest are polylactosamines with 6-linked LacNAc branches on the internal Gal-residues (Galβ1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4GlcNAc-R, also called I-antigens). Erythrocyte glycopeptides carrying such structure were found to be very potent inhibitors of galectin-3 (RL29 and HL-29 at the time11,12) and this structure has only recently attracted attentions as a galectin ligand36. Galectin-1 only binds the terminal LacNAc residue of polylactosamines, which might be more accessible at a cell surface sticking further out35.
The most common O-linked disaccharide Galβ1-3GalNAc (= T-antigen) is a poor binder of galectins -1 and -3 by itself. Yet, it has been presented as a galectin-3 receptor in a number of papers. This, however, is due to a combination of unconvincing binding-assays, combined with the possibility that interaction of the peptide part in subsite E may contribute37,38. In contrast Galβ1-3GalNAc and its 3-sialylated version are good binders of galectin-8N, and maybe preferred ligands for this galectin6. O-linked glycans can also be extended with LacNAc containing moieties (as in core-2, -3, and -4), even with polylactosamines, which may bind galectins the same way as when present in N-glycans33.
The obtained data highlight that “the driving force for the recognition of mucin-type O-glycan glycopeptides by galectins remains largely unknown”38.
Some glycosphingolipid derived glycans of the lactoseries are the same as milk derived free glycans, and relatives of them, and bind galectins according to rules for small saccharides described above, whereas other glycosphingolipid derived glycans violate the core binding rules, and so are poor galectin ligands11,17,18. For example the Galα1-4Gal or GalNAcβ1-4Gal, typical moieties of globoseries and ganglioseries, respectively, will not bind any galectin well. On the other hand, the terminal GalNAcβ1-3Gal in globotetraose and the terminal Galβ1-3GalNAc as found in e.g. GM1 ganglioside may very well bind galectins with preference for these disaccharides, e.g. galectin-8N39,40. On the other hand the glycan of GM1 ganglioside binds moderately to galectin-3 (about like lactose) and not at all to galectin-137. With lactose residue directly linked to ceramide, it is possible that interaction of the latter in subsite E might contribute to very membrane close binding, but this possibility remains largely unexplored. In the GL-Lect-hypothesis18,41 for endocytosis, galectin-3 is thought to first bind N-glycans resulting in cross-linking and somehow exposure of glycosphingolipid binding-sites, but the mechanism and specificity of the latter remains unclear.
When tested in solution intact glycoproteins often have much higher affinity (sub μM) for galectins than their component glycans. This has been shown to be due to monovalent interactions in some cases42,43, and multivalency plays a limited role. Instead additional interaction of the galectin with the protein part of the glycoprotein, e.g. in subsite E or elsewhere, may contribute to affinity, as evidenced by the galectin-3 R186 mutant mentioned above. NMR analysis of galectin-3 suggests that its binding to a glycoprotein (CD146) also engages sites outside the core carbohydrate binding-site (A,B,C,D)44.
The di-GlcNAc, tri-Man core common to all complex of N-glycans is often found near the glycoprotein surface when resolved by X-ray crystallography as exemplified in Fig. 5, indicating good opportunity for added galectin interactions there. Modeling of N-glycans onto a glycoprotein shows great variation of the immediate protein environment of the different N-glycans, giving a complex picture for galectin interactions with many opportunities exemplified in45. A consequence would be that the particular site of the glycan within the glycoprotein would also play a role for affinity, making the study of it very complicated and so far largely unexplored. In a study of galectin-3 effects on endocytosis of cell surface CD98, mutation of one or another of its 4 N-glycosylation sites thereby removing one or another of its four N-glycans, had dramatically different effects31.
Nevertheless the structure of the N-glycans also determines selectivity for galectin binding and ensuing biological function. For transferrin and haptoglobin, binding of galectin-3 or galectin-1, respectively, requires presence of at least triantennary N-glycans as mentioned above, and the bound fractions carrying these have different intracellular fate after endocytosis, compared to the unbound fractions25,30. On the other hand, comparison of galectin binding of EPO-glycoforms from different glycosylation mutant CHO cells, gave less clear cut results23.
Di- or multivalent binding of a galectin to a cell surface, glycoprotein or other ligand can theoretically have two effects – induce cross-linking and/or enhance affinity. Cross-linking is clearly important for many biological effects of galectins, but overall the affinity enhancement by multivalency for galectins is none or modest -- nothing near the glycosidic cluster effects seen for hepatic asialoglycoprotein-receptor described above8. The common statement that “galectins have low monovalent affinity but this is enhanced by multivalency” is largely wrong.
To claim affinity enhancement by multivalency, one needs fair comparison with the relevant monovalent interaction, but this has rarely been done. For example, a multivalent cluster-lactoside was tested as inhibitor in solution, and compared with a monovalent ligand having the same possible interactions with a single galectin CRD, including with extended interaction in subsite E by linkers or protein parts. The larger clusters had only increased inhibitory potency in proportion to the number of lactose moieties, but no per lactose affinity enhancement46. One can also compare the inhibition for monovalent mutants with the wild type galectin. Doing so, showed that a monovalent mutant of galectin-1 had the same affinity for asialofetuin (ASF) as wt galectin-142. In this assay, a low concentration of galectin-1 (0.4 μM) in solution was tested with higher amounts of ASF as inhibitor. This means that the measured Kd will likely represent the first bound galectin molecule, again likely to be monovalent. In a similar assay also monovalent galectin-3 (the CRD lacking the N-terminal domain) had the same affinity as wt galectin-3 for ASF15.
In another type of assay, excess soluble galectin is bound to ligand immobilized on a surface, like in an ELISA-setting, by SPR or to cells. Also in this case, monovalent galectin-1 had same47 or only slightly (~3 fold) lower affinity compared to wt galectin-135,47.
When increasing galectin-3 concentrations are added to a ligand coated surface (or ASF in solution), a cooperative like increase of binding occurs, and this is dependent on the cross-linking N-terminal domain48,49. This galectin-3 self-association has been thoroughly reviewed by Sato in the present series50,51. Here only a few points will be added on its unusual features. This galectin-3 self-association does not result in increased affinity for ligands, as evidence, for example, the same concentrations of lactose inhibited binding of low or high galectin-3 concentrations to laminin49. Instead galectin-3 appears to pile up on itself, that is more galectin-3 molecules bind than the available glycan binding sites. Adding increasing concentration of galectin-3 fails to reach saturation of binding. Adding increasing concentrations of cold galectin-3 will not compete with traces of labeled galectin- 3, as usual in receptor-binding assays, but instead enhance its binding. This occurs on a surface immobilized glycoprotein (like IgE or laminin)34,48,49, on a soluble glycoprotein (like ASF), and on a cell surface43. The self-association of galectin-3 blocks (consumes) carbohydrate-binding sites, even beyond those occupied by glycan ligands. This was directly observed using a fluorescence anisotropy assay with a fluorescein tagged probe specifically binding the galectin-3 carbohydrate recognition site43. CRD-CRD binding was proposed as one explanation, as this is known to be lactose inhibitable, but there may be other explanations.
The galectin binding affinity and number of sites on a cell surface is difficult to determine as binding tends not to be saturated with adding increasing concentration of galectin (see for example23), preventing the usual type of Scatchard analysis. One reason is probably that there are multiple binding sites with a range of affinities for galectins, and another can be the self-association phenomenon of galectin-3 described above. As alternative way, we used the concentration of lactose required to inhibit cell surface binding, to estimate the affinity of galectin-8 to be in the 0.1 μM range and its individual CRDs around 2 μM6. These are also usual concentrations of other galectins giving “decent” binding in FACS analysis in many studies, carried out at 4 ºC to prevent endocytosis.
Using cells with mutation in glycosylation machinery, it is clear that complex N-glycans are major binding sites for all galectins tested, as binding is strongly reduced when the key enzyme Mgat1 is deleted, although the galectin-8 N-terminal domain by itself show sialic acid dependent binding. Over expression of 2-6 linked sialic acid will also strongly reduce binding for most galectins, as expected from specificity for small saccharides described above. However, mutational deletion of Mgat4A and B and Mgat5 required of the different third and fourth antennas of N-glycans seem to not make so much difference for cell surface binding overall22,23. Thus, the selectivity for particular N-glycans described above, is not as clear when testing binding to whole cells at 4 ºC. Indeed, it has been proposed that the overall binding of galectin to cell surface is governed by the number of LacNAc residues rather than their specific position within N-glycans, and said to be bioequivalent52. This may be partially true, as cells appear regulate density of LacNAc residues, and to compensate lack in one context with more in another, for example, by binding to poly-N- acetyllactosamines14,23.
Selectivity may perhaps appear more clearly under in vivo conditions at 37 ºC as discussed in a separate review in this series 53 and some examples described above15,25,30,31,53. Glycoproteins and galectins at a cell surface undergo rapid movements and cycles of endocytosis and exocytosis with relatively short time scales (minutes). To illustrate the complexity, intact galectin-8 requires N-glycans but not 2-3 sialic acid to bind to the cell6,22 surface, but after endocytosis, the strong binding of the N-CRD to 2-3 sialylated galactosides completely determines the intracellular trafficking54. This prompted us to name the PhD thesis of Susanne Carlsson (first author of these papers) “Broad outside, fine inside” referring to specificity. To decipher the complex mosaic of specific galectin interactions in live cells and animals is a big but interesting challenge, beyond the scope of this review.
In a few cases it has been shown that surprisingly low concentrations (nM) of galectins had an effect when added to cells18,55,56. This needs an explanation, and presence of a glycoprotein receptor with low nM affinity for the galectin is one possibility. As evidence for this has not been captured in the more broadly addressed experiments described above, it would also have to be very selective. This interaction could be multivalent, but monovalent interactions can also reach low nM affinities, at least with artificial ligands7,57. Even if very high affinity glycoprotein receptors are found, the abundant galectins would also have a whole range of lower affinity cellular ligands. An intriguing scenario for further investigation.