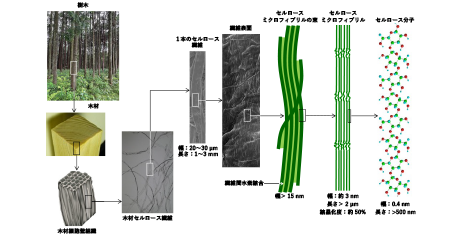
The effective utilization of plant cellulose and its expansion in quantity are highly expected for creation of a sustainable society. This is because cellulose is a renewable material that fixes CO2 in the atmosphere. In various cellulose materials, cellulose nanofibers (CNFs) prepared from plant cellulose fibers are attracting attention as new bio-based nanomaterials. It has already been known that plant cellulose fibers are aggregates of crystalline nanofibers called “cellulose microfibrils (CeMFs).” However, because CeMFs are strongly bound to each other by numerous hydrogen bonds in each cellulose fiber, individual CNFs separated from CeMF bundles have been impossible to prepare and use as nanomaterials. We have reported that fully nano-dispersed CNFs can be prepared by catalytic oxidation of plant cellulose fibers under aqueous conditions at room temperature and normal pressure. In this paper, the method of preparing CNFs is described, and their structures and functions are reviewed with related research achievements. ...and more
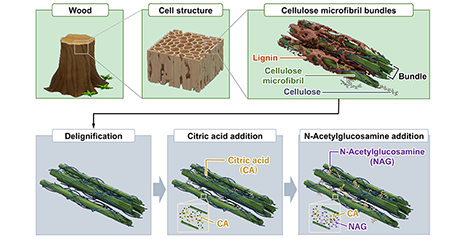
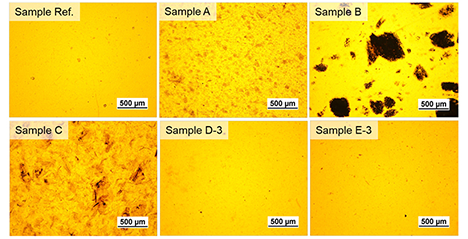
Cartilage is a tissue that does not regenerate itself naturally, and the number of patients with osteoarthritis continues to increase. Current treatment methods have limitations, making the development of new regenerative technologies an urgent task. The author has developed a wood-derived sponge hydrogel that retains the skeletal structure of wood while controlling the amount of hydrogen bonding between cellulose molecules, resulting in stiffness and water content equivalent to that of articular cartilage. Furthermore, this sponge hydrogel exhibits higher compressive strength and better shape recovery (cushioning effect) than cartilage. In vitro tests showed that the hydrogel was non-toxic and promoted the chondrogenic differentiation of human mesenchymal stem cells. In vivo studies demonstrated that after transplantation into a rabbit femoral cartilage defect, cartilage extracellular matrix formed within four weeks, and the defect was completely repaired with hyaline cartilage by week 12. This sponge hydrogel has the potential to overcome the challenges of current treatments and serve as a sustainable cartilage regeneration material derived from forest resources. ...and more
We propose the establishment of a new material cycle utilizing cellulose nanofibers (CNFs) derived from waste materials. We aim to add value to agricultural products, reduce management costs by increasing the efficiency of waste plastic reuse, and improve attractiveness to young people by presenting a new model of greenhouse horticulture. We will form a recycling-oriented sustainable society through Japanese-style agricultural-industrial collaboration. ...and more
Cellulose is the most abundantly reproduced natural biopolymer on Earth. It has recently been attracting attention as a renewable resource. Cellulose is mostly derived from plants and used as a material for various purposes, including pulp production. However, some of the mechanism of its biosynthesis remains to be solved, which markedly varies with the biological origin of the producing strain. Artificial control of the mechanism would enable us to expand the applicability of cellulose. Accordingly, we have been conducting research into the mechanism of cellulose synthesis using acetic acid bacteria, as well as the application of their products. Some of our efforts are described below. ...and more

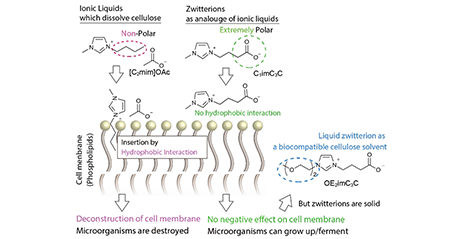
Commercializing second-generation bioethanol derived from cellulose is challenging due to its high crystallinity and resistance to chemical reactions. To overcome this, it is necessary to pretreat cellulose to lower its crystallinity. However, there had been no solvent that can efficiently pretreat cellulose at room temperature and pressure. This had resulted in high energy costs for cellulose pretreatment, leading to a negative energy balance for bioethanol production and consumption. It has been reported that cellulose can be dissolved using salts called ionic liquids, which are liquid at temperatures below 100°C. Since then, ionic liquids have been improved, and it is now possible to dissolve cellulose at ambient temperature and pressure. The cellulose-dissolving ionic liquids typically contains a carboxylate or phosphate anion. This advancement significantly reduces the energy cost required for pretreatment. However, additional reduction of energy costs is necessary due to the low energy density of ethanol. Therefore, the next step is to ensure continuous process. To convert cellulose to ethanol, hydrolysis and microbial fermentation are required, in addition to pretreatment. However, the typical ionic liquids used for pretreatment are highly toxic to microorganisms, such as yeast, which means that the process cannot be completed continuously in a single container (this process is called one-pot bioethanol production). Therefore, in 2017, we have developed a low-toxicity ionic liquid that can dissolve cellulose. ...and more
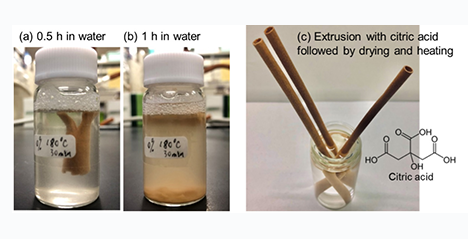
The movement to replace plastic products with environmentally friendly materials is accelerating due to the seriousness of not only global warming but also the problem of plastic waste in the oceans. Biomass resources such as trees and grasses have existed on the earth for a long time and are considered "the ultimate environmentally friendly materials" that can be metabolized by natural processes that clean up the environment. In this article, we introduce the approach of powdering and mixing biomass with cellulose derivatives to confer the property of moldability on biomass without using petroleum-based resins and to generate three-dimensional molded products by extrusion. ...and more
Cellulose is a low-cost and versatile material and has long been used as paper and cloth. With the growing attention to the Sustainable Development Goals, recent studies have endeavored to expand the use of this representative biomass-derived polymer into highly functional materials. This article reviews our recent studies on the self-assembly of cello-oligosaccharides (i.e., short cellulose) for functionalizing conventional cellulose materials. Cello-oligosaccharides can be prepared by hydrolysis of naturally derived cellulose and by chemical synthesis via enzyme-catalyzed oligomerization. The former is a scalable method, and the resultant cello-oligosaccharides can be employed to add nanostructures to cellulose materials via self-assembly. The latter method enables us to synthesize cello-oligosaccharides with functional groups, the self-assembly of which allows for introducing functional groups as well as nanostructures into cellulose materials. ...and more

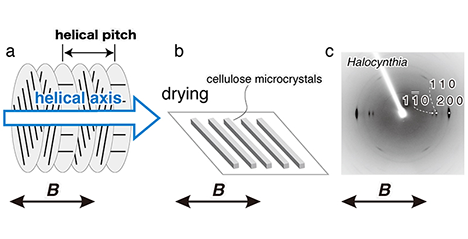
Cellulose microcrystals prepared by treating natural cellulose (cellulose I) with acid are slender rods or whiskers of a few nanometers width and are known to have properties such as high strength, high elastic modulus, and low thermal expansion. It has also long been known that cellulose, an antimagnetic material, has magnetic anisotropy and various studies have been reported on the response of microcrystals of cellulose I to a strong magnetic field. On the other hand, there have been no studies on the magnetic field response of cellulose II, the crystalline form of regenerated cellulose fibers such as rayon. Therefore, this article summarizes the magnetic field orientation behavior of cellulose I microcrystals and introduces our recent efforts on the enzymatic synthesis of cellulose II microcrystals and their three-dimensional orientation using a magnetic field. ...and more
Cellulose is a natural polysaccharide, and is functionally classified as a structural polysaccharide. Its superior strength is attributed to the fact that it is composed of multiple molecular chains, and it has a structure known as cellulose I crystal that exhibits a high crystalline modulus. The fact that such an agglomeration of polymers can be synthesized by enzymatic proteins suggests that cellulose biosynthesis is a mechanism designed to synthesize the strongest possible structure by controlling the polymer chains at ambient temperature and pressure in aqueous solvents. In comparison with the typical formation process for general-purpose polymers, which involves high temperature, high pressure, and harsh solvents, the enzyme cellulose synthase possesses an extremely sophisticated “green” mechanism for controlling polymer structure. In this paper, I will describe efforts to reconstitute the cellulose-synthesizing activity of cellulose synthase, the mechanism of which we have been seeking to elucidate for more than 10 years. ...and more
