
Yoshihiko Amano
Professor, Faculty of Engineering, Shinshu University
He completed his doctor’s program in Bioscience and Textile Technology at the Graduate School of Science and Technology, Shinshu University, to obtain a doctoral degree in engineering.
After serving as a researcher for the Agricultural Technology Institute of Nagano Farmers’ Federation, he was appointed as a Research Assistant at the Faculty of Engineering, Shinshu University, in 1995 and then as an Assistant Professor. He served as the director of the Cooperative Research Center, as well as a councilor, faculty dean, and vice president of Shinshu University.
He worked as a postdoctoral researcher at the University of Texas at Austin and the University of California, Davis, for about 1.5 years.

Masahiro Mizuno
Associate Professor, Faculty of Engineering, Shinshu University
He completed his doctoral course in Biochemistry and Biotechnology at the United Graduate School of Agricultural Science, Tokyo University of Agriculture and Technology, to obtain a doctoral degree in agriculture.
After serving as a Special Researcher of the Japan Society for the Promotion of Science, he became an Assistant Professor in the Faculty of Engineering, Shinshu University, in 2006 and has been in his current position since 2017.
Cellulose is the most abundantly reproduced natural biopolymer on Earth. It has recently been attracting attention as a renewable resource. Cellulose is mostly derived from plants and used as a material for various purposes, including pulp production. However, some of the mechanism of its biosynthesis remains to be solved, which markedly varies with the biological origin of the producing strain. Artificial control of the mechanism would enable us to expand the applicability of cellulose. Accordingly, we have been conducting research into the mechanism of cellulose synthesis using acetic acid bacteria, as well as the application of their products. Some of our efforts are described below.
Cellulose is said to be the most abundantly produced organic biopolymer on Earth because it is photosynthesized as the major component of the cell walls of plants. On the other hand, some bacteria produce and skillfully use cellulose. The cellulose produced by bacteria is called bacterial cellulose (BC), which is distinct from plant-derived cellulose.
In 1886, A. J. Brown reported the presence of cellulose in culture broths of Acetobacter xylinus, an acetic acid bacterium1. Since then, research into cellulose biosynthesis by bacteria has been conducted with a focus on A. xylinum (presently known as Komagataeibacter xylinus, but herein called Gluconacetobacter xylinus following long-standing nomenclature). One of the major reasons for this focus was that G. xylinus produces cellulose in amounts allowing extensive structural analyses. Thereafter, the set of genes for cellulose biosynthesis has been increasingly analyzed using molecular biological techniques, and various models of cellulose biosynthesis have been proposed based on the correlations between genes encoding actual structural information for cellulose. On the other hand, recent advances in genomic analysis technology have enabled us to conduct genomic DNA analysis quicker and more easily. As a result, a number of genes encoding proteins homologous to cellulose synthase were discovered in the genomic DNA of many bacterial species, irrespective of the availability and quantity of actual cellulose production.
Eubacteria can be roughly divided into two groups based on Gram staining patterns stemming from cell wall structural differences: Gram-positive and Gram-negative bacteria. Regarding Gram-positive bacteria, which have a cell wall with well-developed peptide glycan layer, there have been almost no reports of models of cellulose biosynthesis since the 1960s, when substantial research into bacterial cellulose biosynthesis began with a report in Sarcina ventriculi. However, a model was reported in Rhodococcus sp. MI 2, an actinomycete, in 20132. Among the Gram-negative bacteria, which have a thin peptide glycan layer, on the other hand, cellulose-producing bacteria are found in two groups: the phylum Cyanobacteria and the phylum Proteobacteria. The members of the phylum Cyanobacteria (blue-green algae) produce oxygen by photosynthesis. Cyanobacteria are known to produce a highly diverse range of extracellular polymeric substances (EPSs), presumably for protection against drought and ultraviolet exposure, as well as for adhesion and control of mobility of cells. While it had long been suggested that cellulose is one of the EPSs, Nobles et al. demonstrated the actual presence of cellulose using electron microscopy and instrumental analysis in 20013.
The phylum Proteobacteria is a large taxonomic group of eubacteria and contains most of the cellulose-producing bacteria that have been reported so far. Proteobacteria are further divided into six classes, including alpha, beta, and gamma proteobacteria, in which cellulose-producing bacteria are found. The class Alphaproteobacteria includes the family Acetobacteraceae, to which the photosynthetic bacterium Rhodobacter sphaeroides belongs. The structure of its complex of cellulose synthases (BcsA) and cellulose synthesis-related protein (BcsB) was elucidated by X-ray crystallography4. Additionally, many cellulose-producing bacteria have been reported among the enterobacteria in the gamma subdivision of the Proteobacteria. While forming a biofilm, many of the bacteria belonging to the order Enterobacterales do not produce cellulose as a simple substance but rather cellulose as a component of EPSs.
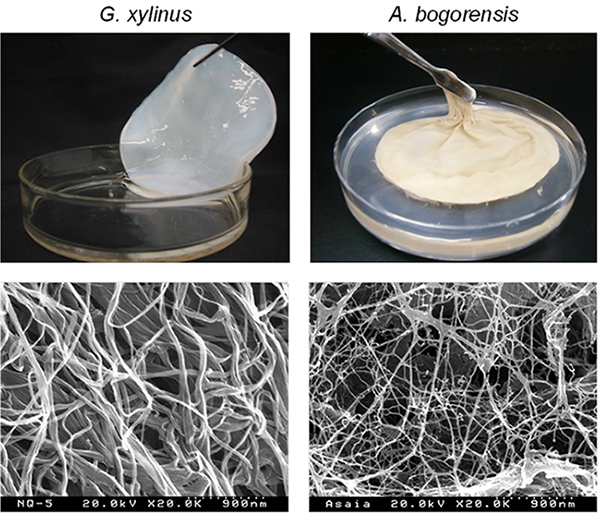
Our laboratory searched for cellulose-producing microorganisms other than G. xylinus, and found that Asaia bogorensis, an aerobic Gram-negative bacteria with peritrichous flagella, produced a cellulose-containing membrane (pellicle) at the gas-liquid interface of the culture broth5. G. xylinus forms firm mat-like pellicles, whereas A. bogorensis forms soft, brittle, thin pellicles (upper panel of Figure 1). A. bogorensis produces less cellulose than G. xylinus. In addition, the cellulose produced by the former contains large amounts of impurities, making it difficult to obtain a white translucent state, like that of nata de coco, with ordinary alkaline cleaning alone. Electron microscopic examination of the fine structure of cellulose fibers after pellicle purification showed that the cellulose produced by A. bogorensis had thinner fibers (approximately 5 nm wide [lower panel of Figure 1]) than that produced by G. xylinus. We confirmed that this pellicle contains cellulose as it is degraded to cellobiose by cellulase. Additionally, the FT-IR and 13C-NMR spectra of its dry state were found to have the same waveform as that of the cellulose produced by G. xylinus.
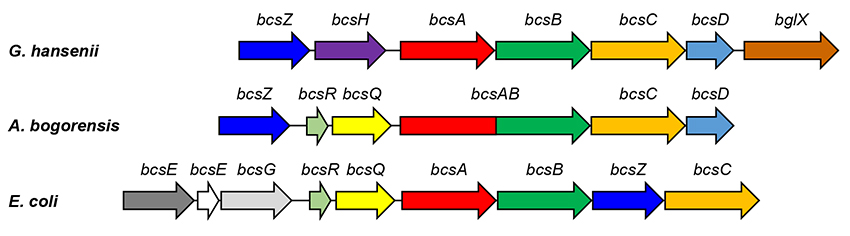
Next, we analyzed the genes involved in cellulose synthesis by A. bogorensis, the results showing that a set of bacterial cellulose synthase operons is present in the genomic DNA. In recent years, bcs operons have been found in many microorganisms. Although A. bogorensis has a bcs operon that is very similar to that of G. xylinus, it shares some features with Escherichia coli and other bacteria (Figure 2). As for the amount of cellulose synthesized, A. bogorensis synthesizes lower amounts than G. xylinus but higher amounts than E. coli and other bacteria; cellulose can be isolated from its pellicles in sufficient amounts for analysis. As such, A. bogorensis appears to have properties intermediate between G. xylinus with high BC production and other microorganisms with low BC production, making it a microbial species of great interest for elucidating the mechanism of cellulose biosynthesis.
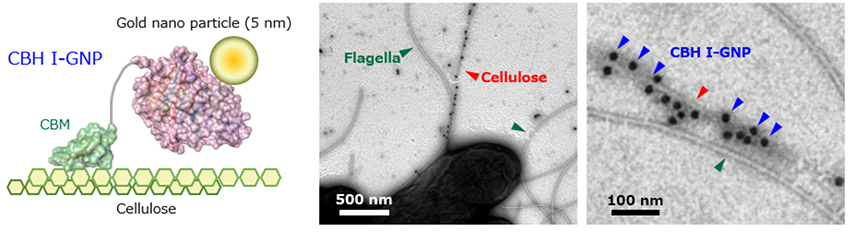
At present, no reason for the thinness of the cellulose fibers of A. bogorensis has been identified. Since the scanning electron microscope image shown in Figure 1 was taken to view cellulose fibers in the resulting pellicle, we performed transmission electron microscopy (TEM) to reveal the morphology of cellulose fibers immediately after secretion from the bacterial cells (Figure 3). Because A. bogorensis has peritrichous flagella, we used CBH I-GNP, prepared by labeling cellobiohydrolase (CBH I), which has a cellulose-binding module (CBM) that specifically adsorbs to cellulose, with gold nanoparticles (GNP). As a result, we identified fibers with specific adsorption of CBH I-GNP and successfully distinguished between the flagella and cellulose fibers. Additionally, the cellulose fibers of A. bogorensis were found to be much thinner than those of G. xylinus, measuring approximately 5-20 nm in width already at the time of discharge from bacterial cells.
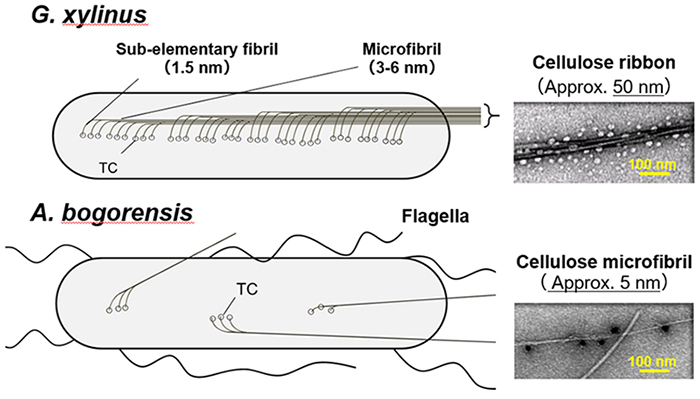
The shape of cellulose fibers produced by organisms has been found to be associated with the arrangement of cellulose synthase complex (terminal complex: TC) units localized on the cell membrane. Inder Saxena et al. analyzed the sequence of the TC in G. xylinus using the freeze-fracture method and reported that the TC units were arranged in a row on the bacterial cell intima (Figure 4)6. In view of these facts, we assume different arrangements of TC units on the bacterial cell intima. Judging from their thinness, the cellulose fibers produced by A. bogorensis are thin at the level of several TC units; therefore, we consider that the nanofiber is composed of several sub-elementary fibrils. A schematic diagram is shown in Figure 4.
Since plant-derived cellulose is produced as a component of the plant cell wall, it is extracted as pulp while mechanical and chemical decomposition of other components is occurring to break its higher-order structure. BC, on the other hand, is produced as nano-level cellulose and forms a large structure with cellulose fibers interwoven in a mesh-like form according to the movement of bacterial cells. Hence, it is possible to make a three-dimensional cellulose shape by controlling the movement of cellulose-producing bacteria. Applying the advantages of the simple structure with pure cellulose alone and the three-dimensional network structure, in combination with the free manufacture into any designed shape, a wide variety of functional cellulose materials have been reported worldwide. Most simply, spherical or floriform cellulose can be obtained by rotation culture. In this paper, we describe the production of tubular BC.
As described above, acetic acid bacteria produce cellulose in gas-liquid interfaces. Accordingly, we conceived the use of a gas-permeable but liquid-impermeable porous Teflon tube as a culture vessel. When an acetic acid bacterial culture broth in suspension in a fresh medium was placed in a Teflon tube, and the tube was sealed and subjected to standing culture in a humidified vessel, a BC tube was produced along the inner wall of the Teflon tube (Figure 5).
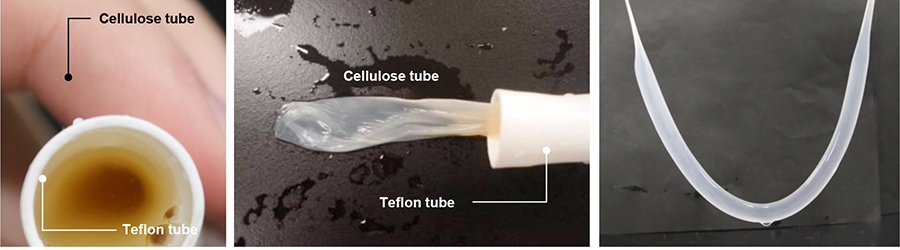
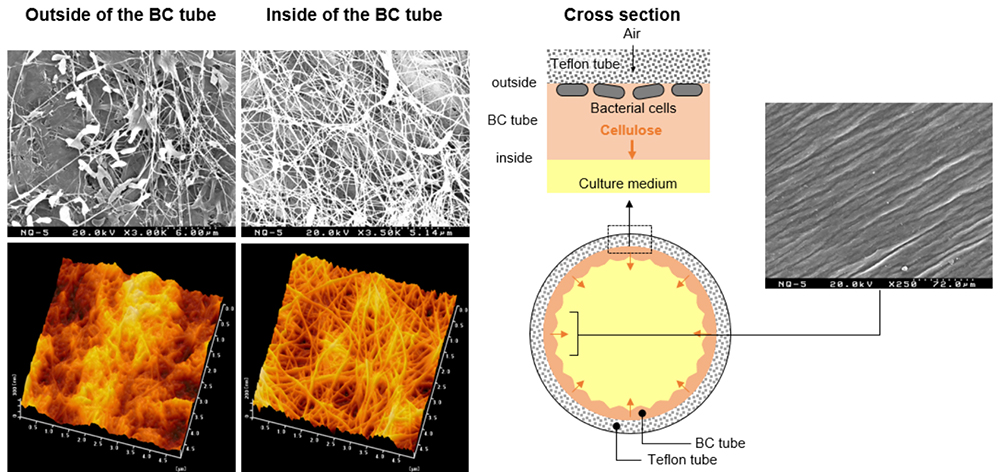
Analysis of the surface structure of a BC tube obtained using the present method revealed structural differences between the inner and outer surfaces of the BC tube (Figure 6). A clean network was formed by cellulose fibers on the inner surface of the BC tube, with only sparsely distributed bacterial cells. On the outer surface of the BC tube, a network of cellulose fibers was found only partially, with collapsed meshes. Although cellulose-synthesizing acetic acid bacteria have affinity for water, they were well adsorbed to slightly hydrophobic surfaces. In addition to the gas permeability of Teflon tubes, the hydrophobic surfaces acted as scaffolds for acetic acid bacteria to produce cellulose with the bacterial cells immobilized on the tube wall. As a result, the movements of acetic acid bacteria may be suppressed between the Teflon tube and cellulose as cellulose production progresses, preventing the formation of clean woven structures. When examining the inner surface of the BC tube at low magnification, furrowed streaks were observed. This was attributable to the localization of bacterial cells on the outer surface of the BC tube; the first-formed membrane was pushed inward causing its radius to decrease and resulting in wrinkling. As described above, the BC tube obtained using the present technique was structurally unique as its inner and outer surfaces have different structures and properties.
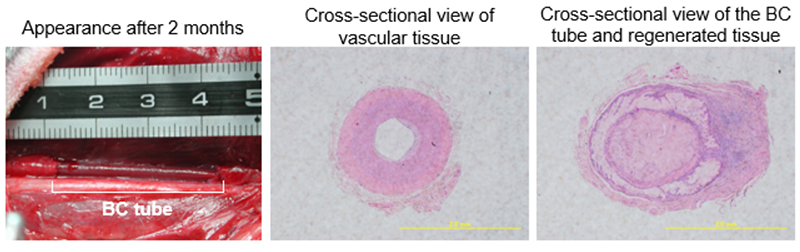
We then considered applying BC tubes as vascular grafts. Outside Japan, Klemm et al. created BC tubes for application as vascular grafts using a method distinct from ours7. They created thick BC tubes using an approach that was completely different from ours, and reported that their BC tubes might serve as vascular grafts. Our study included animal experiments in cooperation with Dr. Kanemaru at the Department of Otolaryngology, Faculty of Medicine, Kyoto University. A carotid artery of a beagle dog was replaced with a BC tube and dissected 2 months later, as shown in the left panel of Figure 7. The blood vessel was partially replaced with an approximately 5 cm BC tube prepared using a Teflon tube having an inside diameter of 6 mm, and the animals were reared under ordinary conditions for 2 months. The tube functioned normally, without forming any thrombus in the blood vessel. Thereafter, ordinary vascular tissue and an embedded BC tube were examined (Figure 7, center and right panels). Ordinary vascular tissue is thick. Although the BC tube was smaller in membrane thickness, it was considered to have sufficient strength. The prepared BC tube was spun into a thread, which was tested for tensile strength and found to be 2 to 3 times stronger than nylon 66 filaments. From this finding, the BC tube was judged not to be problematic for use as a vascular tissue surrogate. Additionally, the surfaces of the BC tube appeared to have regenerated vascular tissue. Therefore, the BC tube may be useful as a scaffold for animal cell regeneration. In fact, the experiments on osteoblast regeneration that we conducted in cooperation with Professor Abe at the Faculty of Textile Science and Technology, Shinshu University, showed good proliferation of osteoblasts on the BC surfaces, suggesting that calcium deposition for differentiation might occur earlier than in control culture using hydrophilic dishes. Therefore, nanocellulose-based cellulose materials are considered to have high affinity for animal cells. A study conducted outside Japan reported that such a material promoted skin regeneration as a wound-dressing8, and its use in the healthcare field is attracting attention. However, there is a high hurdle to the regulatory approval of such medical applications, so further investigation is needed.
There have been a variety of reports on the application of BC, making the best use of its functional features such as high strength, hydrophilicity, and biocompatibility. Gel-like membranes, called pellicles, obtained by static culture of BC, are used in foods such as nata de coco and also used as wound dressings. The BC tube described in the previous section may be viewed as an application of pellicles. Although pellicles are sometimes used after defibering, this process often requires special equipment and energy. We discovered that a defibered slurry containing nano-sized fibers was readily obtained by mixing tamarind seed gum, which is based on xyloglucan, with BC pellicles, and processing the mixture in a home mixer9. We also found that this slurry was adsorbable onto hydrophobic substrates such as plastics and capable of hydrophilizing hydrophobic surfaces. Although its detailed mechanism still needs to be investigated, BC fiber refinement is expected to be a new application of defibered BC.
BC surface modifications made by introducing various functional groups into the hydroxyl group of cellulose are being considered. We successfully introduced an oligo-DNA molecule by amide linkage to a tetramethylpiperidine-1-oxyl (TEMPO)-oxidized cellulose as prepared by carbonylating the 6-hydroxyl group of cellulose10. The thus-introduced DNA molecule was found to be complementary to, and dissociated from, an oligo-DNA molecule having a complementary sequence, in a temperature-dependent manner. However, the modified DNA molecule behaved in a different way in terms of the temperature dependency of double-strand formation and cleavage, compared with the DNA molecule in solution. Because cellulose can become associated at the molecular and microfibril levels, the present complex is capable of controlling the molecule’s conformation and dispersion status under various conditions. This is an interesting observation.
As described above, BC with its distinct characteristics is considered to be potentially useful for a wide range of applications. However, its current availability is limited to relatively high-value-added fields. Applications in a broader range of fields are expected with cost reductions by mass-production in the future.
A large number of graduates were involved in the conduct of the present study. We thank our external partners for their collaboration. For the biological applications discussed in the present study, in particular, we would like to express our profound gratitude to Dr. Kanemaru, who was affiliated with the Faculty of Medicine, Kyoto University, in those days.