
Kosuke Kuroda
Associate Professor, Department of Bioscience and Biotechnology, Kanazawa University
He received his Ph.D. in Sep. 2014 from Tokyo University of Agriculture and Technology. After two years as a research assistant professor and three years as an assistant professor at Kanazawa University, he started his current position in March 2021.
I am enjoying the freedom of ionic liquid world and creating new solvents every day. I am always excited because nobody knows the expressed properties of new ionic liquids at all, until I actually synthesize them.
http://ionicliquid.w3.kanazawa-u.ac.jp
Commercializing second-generation bioethanol derived from cellulose is challenging due to its high crystallinity and resistance to chemical reactions. To overcome this, it is necessary to pretreat cellulose to lower its crystallinity. However, there had been no solvent that can efficiently pretreat cellulose at room temperature and pressure. This had resulted in high energy costs for cellulose pretreatment, leading to a negative energy balance for bioethanol production and consumption.
It has been reported that cellulose can be dissolved using salts called ionic liquids, which are liquid at temperatures below 100°C1. Since then, ionic liquids have been improved, and it is now possible to dissolve cellulose at ambient temperature and pressure. The cellulose-dissolving ionic liquids typically contains a carboxylate or phosphate anion2,3. This advancement significantly reduces the energy cost required for pretreatment. However, additional reduction of energy costs is necessary due to the low energy density of ethanol.
Therefore, the next step is to ensure continuous process. To convert cellulose to ethanol, hydrolysis and microbial fermentation are required, in addition to pretreatment. However, the typical ionic liquids used for pretreatment are highly toxic to microorganisms, such as yeast, which means that the process cannot be completed continuously in a single container (this process is called one-pot bioethanol production). Therefore, in 2017, we have developed a low-toxicity ionic liquid that can dissolve cellulose4.
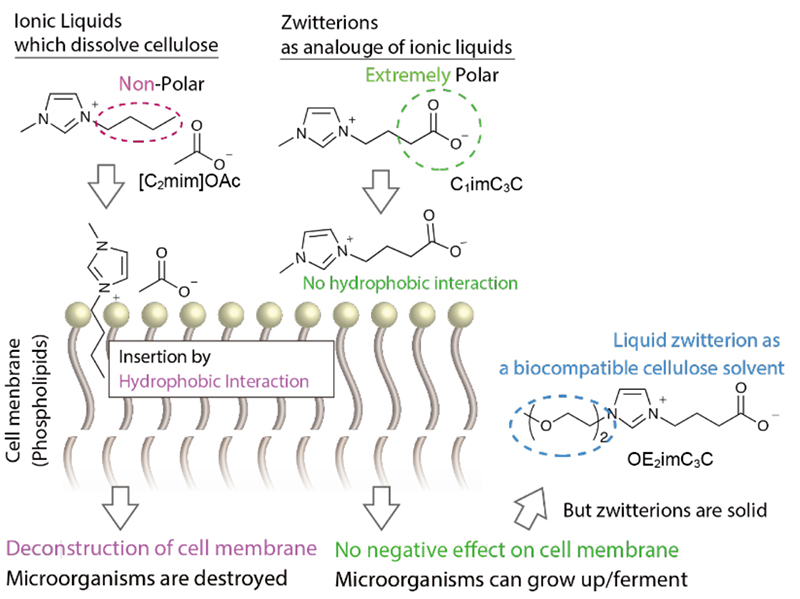
The toxicity mechanism of ionic liquids to microorganisms is as follows (Fig. 1 left)5. The cations of the ionic liquids are attracted to the phosphate anions of the phospholipids. Additionally, the alkyl chain of the cation is inserted into the lipid moiety through hydrophobic interactions. Consequently, cations accumulate in the cell membrane, leading to its eventual collapse. Therefore, we developed a strategy to decrease the hydrophobic interaction between the cationic alkyl chain and the cell membrane, which reduces toxicity (Fig. 1 center). To achieve this, we introduced a highly polar anion at the end of the alkyl chain of the cation, resulting in a zwitterion structure where no hydrophobic alkyl chain exists.
The zwitterions are more easily solidified than ionic liquids and are basically solids at temperatures below 100 °C. The synthesis of a liquid carboxylate-based zwitterions, i.e. zwitterionic liquids, was based on the report by Fujita et al. that "the introduction of an oligoether into a sulfonate-based zwitterion results in a liquid at around room temperature"6. Whereas the melting point of a typical zwitterion (C1imC3C, structure in Fig. 1 center) was above 100 °C, the oligoether-introduced zwitterionic liquid (OE2imC3C, structure in Fig. 1 right) had no melting point and a glass transition point observed at -62 °C. Later, it has been confirmed that the supercooled state is highly stable and solidifies at room temperature after approximately one month. It has an actual melting point of 64°C7, but remains in a liquid state at room temperature for a long term. OE2imC3C dissolved up to 6 wt% cellulose at 100°C.
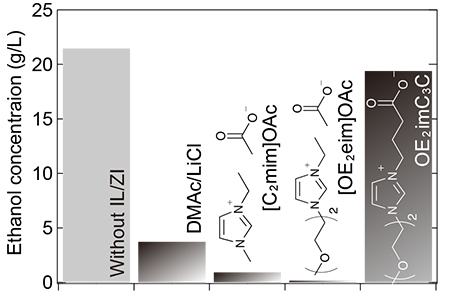
We studied through fermentation using E. coli KO11, which is capable of ethanol fermentation. Fermentation of glucose in a medium without OE2imC3C produced 21.5 g/L of ethanol (Fig. 2). The addition of 0.5 mol/L OE2imC3C resulted in 19.4 g/L of ethanol, with little inhibition of the fermentation. However, when 0.5 mol/L of ionic liquids ([C2mim]OAc, [OE2eim]OAc, structures shown in Fig. 2) were added, the ethanol concentration was only about 1 g/L. OE2imC3C enabled both cellulose solubility and efficient ethanol fermentation.
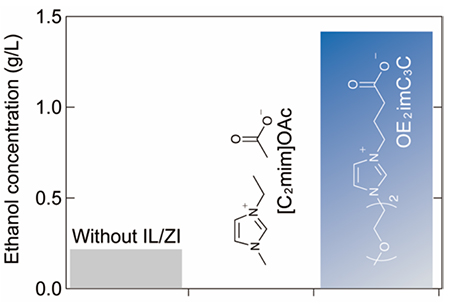
Bagasse, the residue from sugarcane processing, was converted to ethanol in a one-pot process. The bagasse was pre-treated with OE2imC3C at 120°C, then added to an acetate buffer (OE2imC3C concentration: 0.5 mol/L), hydrolyzed, and fermented by cellulase and E. coli KO11. The use of OE2imC3C resulted in the production of 1.41 g/L of ethanol. In contrast, when [C2mim]OAc was used, the E. coli were killed and no ethanol was produced. Ethanol was also produced without pretreatment, but at a concentration of only 0.2 g/L. The experimental results indicate that OE2imC3C facilitated one-pot ethanol conversion4,8.
OE2imC3C is an excellent low-toxicity cellulose solvent. However, its high viscosity is a drawback. At room temperature, OE2imC3C is a highly viscous liquid, more than syrup. Its viscosity at 80°C was 935 cP, about 1000 times higher than that of water at room temperature. The viscosity increased further when cellulose was dissolved, and the stirring bar stopped turning when 7 wt% cellulose was added.
To address this issue, a co-solvent was utilized. It is known that the addition of an organic solvent to typical ionic liquids can reduce viscosity and facilitate the dissolution of cellulose9. In this case, dimethyl sulfoxide (DMSO) was used. The viscosity was significantly reduced by adding 40 wt% DMSO, which allowed for the dissolution of 14 wt% of cellulose. The OE2imC3C/DMSO mixture was also found to have low toxicity. These results indicate that the cellulose dissolution capacity was improved with the addition of DMSO, without an increase in toxicity8.
Zwitterionic liquids are effective solvents that possess the properties of cellulose solubility and low toxicity. Since they have a short history, there is still room for improvement. We will propose further improved zwitterionic ionic liquids for achievement of one-pot bioethanol production.