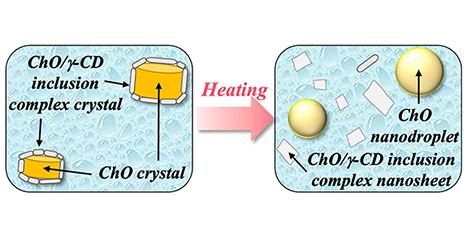
Pickering emulsions, in which cyclodextrin (CD) inclusion complex crystals precipitate at particle interfaces, have attracted considerable attention. In particular, Pickering emulsions composed of phytosterol ester (PSE) and γ-CD have been shown to be effective for masking capsaicin and controlling drug release. However, previous reports have focused on particles larger than 1 μm, and did not sufficiently investigate nanoparticle formation. In this study, we employed cholesteryl oleate (ChO), the main component of PSE, and optimized preparation conditions to successfully obtain ChO/γ-CD nanoparticles. These nanoparticles exhibited a unique core–shell structure, with a ChO crystal core surrounded by multiple nanosheets of ChO/γ-CD inclusion complex crystals forming the shell. Furthermore, the nanoparticles demonstrated dual-stimulus responsiveness, undergoing structural changes upon heating and exposure to bile components. Because they are prepared from inexpensive, safe, and easily handled raw materials, these nanoparticles hold promise as practical stimulus-responsive carriers for applications across diverse fields. ...and more
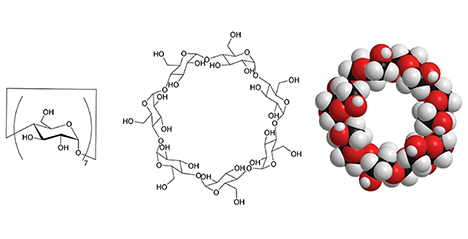
Cyclodextrins (CDs) are cyclic oligosaccharide molecules with a size of 1 nm, consisting of glucose units. This molecule has the property of inclusion, i.e., it allows a variety of molecules to enter the cavity of its ring structure. It not only is the subject of academic study but also is employed extensively in practice in a number of industries, including food, cosmetics and pharmaceuticals. Another salient feature is that these molecules have a number of hydroxy groups. In general, it is possible to convert hydroxy groups into various functional groups through chemical modification. This allows the synthesis of CDs with novel structures and capabilities. Here, we describe the work to create antibacterial CD derivatives by introduction of cationic and hydrophobic groups to disrupt bacterial cell membranes. ...and more
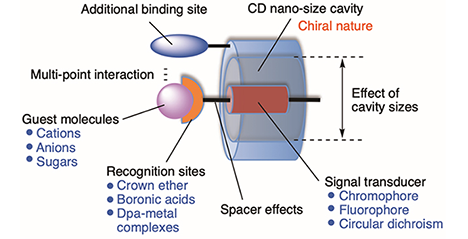
Supramolecules based on non-covalent interactions can rearrange in response to changes in the environment and change their function as complexes. We have been developing supramolecular analytical reagents that focus on the dynamic molecular recognition function of these supramolecules. In particular, we have developed molecular recognition systems based on the inclusion ability of cyclodextrins (CDs: a hexamer is called α-CD, a heptamer is called β-CD, and an octamer is called γ-CD). The inclusion ability of CDs is, in turn, based on the structural changes in the CD cavity of molecular recognition probes, such as metal ion recognition, phosphate derivative recognition, and sugar recognition, as well as the creation of supramolecular chirality built on the chirality of CD cavities derived from natural products (Fig. 1). We have also succeeded in developing spherical ultrasmall CD nanogels with uniform CD orientation by using cationic amphiphilic compounds as emulsifiers, and have revealed their excellent inclusion capabilities. In this review, the development of these CD-based supramolecular analytical reagents is summarized. ...and more
Polyrotaxane is a general term for supramolecules in which a linear polymer chain threads into the cavities of numerous cyclic molecules, and bulky sealing groups are introduced at both ends to prevent the cyclic molecules from dethreading. Therefore, although polyrotaxane is a high molecular weight compound, there are no covalent bonds between the numerous cyclic molecules and the linear polymer chain, and these molecules can be regarded as mechanically linked to each other. One of the cyclic molecules that have been used in polyrotaxane design over the past several decades is a cyclic oligosaccharide, popularly known as cyclodextrin.
In 1993, we began research to design biomaterials with new functions using a polyrotaxane skeleton composed of cyclodextrins, and have reported the emergence of many biomaterials with new functions. All of these functions are supported by the unique characteristics of polyrotaxanes, in which molecules are linked to each other by mechanical bonds, and we are proud to have demonstrated the breadth and depth of a new world that is completely different from that of conventional polymers, in which molecules are linked together by covalent bonds. In this article, we will focus on the following two areas of polyrotaxane research: (1) modulation of cellular functions by molecularly mobile polyrotaxane surfaces and (2) potential application of degradation-responsive polyrotaxane for the treatment of intractable metabolic diseases. ...and more

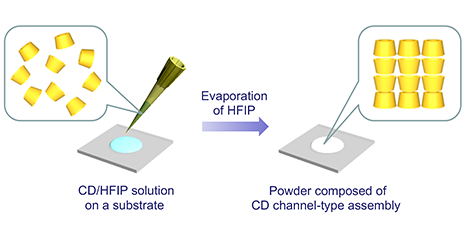
Cyclodextrins (CDs) are a class of cyclic oligosaccharides consisting of several α-(1,4)-linked D-glucopyranose units (Figure 1A). CDs composed of 6, 7, and 8 glucosidic units are called α-, β-, and γ-CDs, respectively, and have been used extensively. They have a sub-nanometer-sized cavity that can accommodate guest molecules of the appropriate size and shape. The inclusion ability of CD has been academically studied as a catalyst, sensor, etc., and has been widely utilized industrially in foods, cosmetics, and pharmaceuticals. On the other hand, CDs can regularly assemble intermolecularly through hydrogen bonding between hydroxyl groups on the upper and lower rims of their doughnut-shaped rings or through host-guest molecular interactions. Recently, much attention has been paid in the fields of supramolecular chemistry and materials science to studies on nano- and microstructures formed by the assembly of CDs5,6. This article reviews the preparation of supramolecular structures by using the regular assembly of CD molecules, and the morphological control of supramolecular structures. ...and more
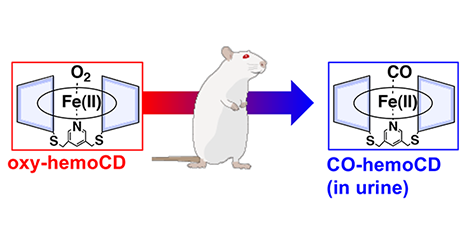
Cyclodextrins (CDs), cyclic oligosaccharides, are water-soluble host molecules that can include various kinds of guest molecules utilized not only in basic research but also as food additives. CDs with oligo-glucose structure have interesting properties; however, CDs can be chemically modified and thereby acquire new functions. We are studying the artificial hemoglobin “hemoCD”, which consists of a per-O-methylated CD dimer and iron porphyrin (FeTPPS). In this article, I describe the strategy of hemoCD preparation and its medical applications. ...and more
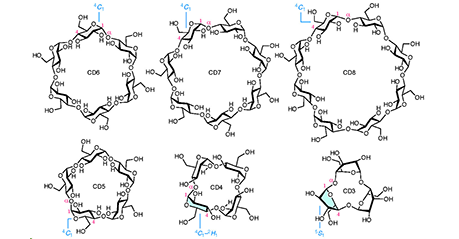
Cyclodextrins (CDs) are cyclic oligomers of α-1,4-D-glucopyranoside. Because the central cavities of CDs can be used to encapsulate small molecules, cyclic hexamer to octamer (CD6–8) have been widely used. While CD-hundreds are known for large CDs, the synthesis of the only CD5 had been only one reported for small CDs. Smaller CDs, such as CD4 and CD3, have never been synthesized because their molecular sizes are too small for the pyranose ring to adopt a stable chair-type conformation. In this report, we describe the chemical synthesis of both CD4 and CD3. The development of a specific bridging group between the 3- and 6-oxygen positions (O-3 and O-6) of D-glucose led to the successful synthesis of these compounds. In other words, the adoption of this bridging group provides both the stereoselective glycosylation reaction and the supple conformation of the pyranose ring, which are required for the synthesis of CD4 and CD3. ...and more