
Nobuhiko Yui
Dr. Nobuhiko Yui received his Ph.D. in engineering from Sophia University (1985) and joined the Biomedical Engineering Research Institute, Tokyo Women's Medical University as an assistant professor, where he was engaged in biomaterials research under Professor Yasuhisa Sakurai. After a postdoctoral fellowship under Professor Jan Feijen at the University of Twente in the Netherlands (1988–89), he moved to the Japan Advanced Institute of Science and Technology (JAIST) as an associate professor in 1993 and started his own laboratory, where he has been leading supramolecular biomaterials research since 1998 when he was promoted to professor. In 2011, he moved to Tokyo Medical and Dental University as a professor in the Department of Organic Biomaterials, Institute of Biomaterials and Bioengineering, where he further promoted the supramolecular biomaterials research he had advocated through interdisciplinary collaboration with engineers, scientists, physicians, and dentists. In 2023, he became Professor Emeritus at Tokyo Medical and Dental University, and in 2018–2020 he became President of the Japanese Society for Biomaterials, contributing to the advancement of research and education on biomaterials science in Japan. He has published more than 400 original papers, reviews, and books, and supervised the research of more than 40 Ph.D. candidates.

Atsushi Tamura
Dr. Atsushi Tamura received his Ph.D. in engineering in 2010 from the Department of Materials Science and Engineering, Graduate School of Pure and Applied Sciences, University of Tsukuba, where he was engaged in research on polymeric biomaterials under the supervision of Professor Yukio Nagasaki. After obtaining his degree, he joined the Institute for Advanced Biomedical Sciences, Tokyo Women's Medical University as a postdoctoral researcher, where he engaged in research on regenerative medicine. In 2011, he joined the Department of Organic Biomaterials, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University as a specially appointed assistant professor, and has been engaged in research on polyrotaxane as a biomaterial. He has been promoting research on the application of polyrotaxane to the fields of medicine and dentistry.
Polyrotaxane is a general term for supramolecules in which a linear polymer chain threads into the cavities of numerous cyclic molecules, and bulky sealing groups are introduced at both ends to prevent the cyclic molecules from dethreading. Therefore, although polyrotaxane is a high molecular weight compound, there are no covalent bonds between the numerous cyclic molecules and the linear polymer chain, and these molecules can be regarded as mechanically linked to each other. One of the cyclic molecules that have been used in polyrotaxane design over the past several decades is a cyclic oligosaccharide, popularly known as cyclodextrin.
In 1993, we began research to design biomaterials with new functions using a polyrotaxane skeleton composed of cyclodextrins, and have reported the emergence of many biomaterials with new functions. All of these functions are supported by the unique characteristics of polyrotaxanes, in which molecules are linked to each other by mechanical bonds, and we are proud to have demonstrated the breadth and depth of a new world that is completely different from that of conventional polymers, in which molecules are linked together by covalent bonds. In this article, we will focus on the following two areas of polyrotaxane research: (1) modulation of cellular functions by molecularly mobile polyrotaxane surfaces and (2) potential application of degradation-responsive polyrotaxane for the treatment of intractable metabolic diseases.
Biomaterial surfaces in contact with the living body are required to have various properties, such as biocompatibility to prevent foreign body reactions and ability to regenerate tissue through modulation of cellular functions. In a previous study, we pointed out that the molecular mobility of biomaterial surfaces may determine the function of cells to which they are attached1. Therefore, we hypothesized that molecularly mobile surfaces may enable flexible control of cell and tissue functions, and to test this hypothesis, we designed polyrotaxane-coated surfaces with the potential to control molecular mobility over a wide range and examined the effects of this molecular mobility on cells. As mentioned earlier, the molecular mobility of polyrotaxane is a structural property based on mechanical molecular linkage.
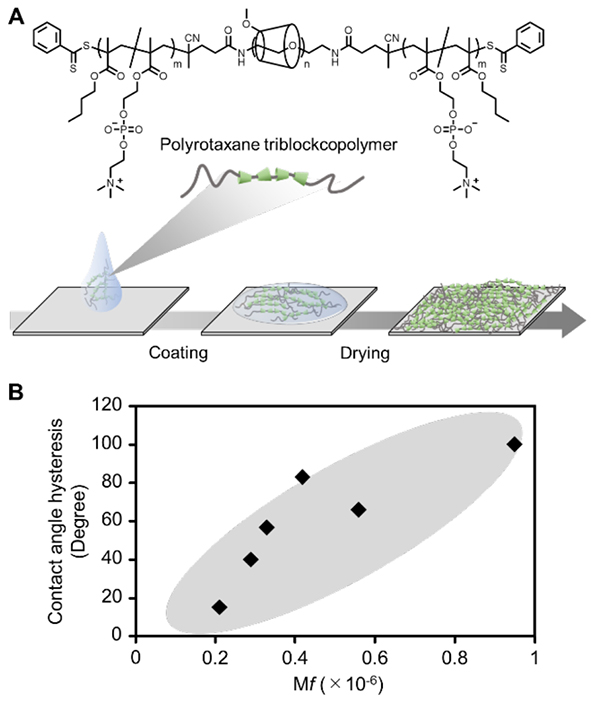
Polyrotaxane surfaces can be prepared by coating the surfaces of glass, metal, plastic, and other vessel materials with a polyrotaxane triblock copolymer to which hydrophobic polymer chains are attached at both ends (Figure 1A)2-5. The degree of molecular mobility also can be adjusted by changing the number of penetrations of α-cyclodextrin and the number of functional groups (e.g., methyl groups) to be modified on α-cyclodextrin. Using the amount of energy loss (ΔD/Δf) per adsorbed amount of polymer in hydrated or dry states, obtained by the quartz crystal microbalance (QCM-D) measurement method, as a measure of surface molecular mobility (mobility factor, Mf), we found that the Mf value tended to be higher for surfaces with fewer α-cyclodextrin penetrations and more methyl group modifications. The polyrotaxane surface had a higher Mf value than surfaces coated with a random copolymer with functional groups similar to those of the polyrotaxane triblock copolymer used (non-polyrotaxane surface). When the difference in contact angle of water in water and air (contact angle hysteresis) was analyzed using these surfaces, hysteresis of more than 100° was observed for the polyrotaxane surface with high molecular mobility, and there was a positive correlation between Mf value and contact angle hysteresis (Figure 1B)3,5. The contact angle hysteresis indicates that the material surface changes dynamically in response to the environment, supporting the idea that polyrotaxane surfaces with higher Mf values (higher molecular mobility) are more environmentally responsive.
The initial adhesion of the cell-adhesive polypeptide sequence arginine-glycine-aspartic acid (RGD) to human umbilical vein-derived vascular endothelial cells was observed using polyrotaxane surfaces modified with α-cyclodextrin (RGD-modified polyrotaxane surface) and a surface coated with a random copolymer containing an RGD group (RGD-modified polymer surface)7, and cell adhesion was observed to be more rapid on the RGD-modified polyrotaxane surface. This suggests that the molecular mobility of polyrotaxane dramatically enhances the multivalent interaction between RGD and integrins. Interestingly, although the RGD-modified polyrotaxane surface enhanced initial cell adhesion, it tended to suppress cell elongation and the formation of actin fibers, which are cytoskeletal proteins. In general, materials with excellent cell adhesion tend to promote cell extensibility and proliferation. However, the results suggest that there is no such a reciprocal relationship on the polyrotaxane surface. When cell extensibility was analyzed on polyrotaxane surfaces with different molecular mobility, the surfaces with higher molecular mobility tended to inhibit cell extensibility, while the surfaces with lower molecular mobility tended to promote cell extensibility. In other words, polyrotaxane surfaces have the potential to independently regulate cell extensibility through molecular mobility while maintaining high cell adhesion.
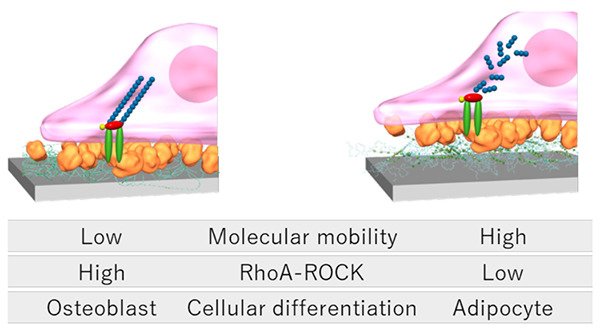
The control of cell extension morphology has received considerable attention in recent decades as a new field of biomaterials research known as mechanobiology. Mechanobiology is the study of how cells and tissues perceive forces generated inside and outside of cells and how these forces affect their activities8. Numerous reports have shown that cells recognize the elastic modulus and nanostructure of adhering vessels, thereby altering their morphology and differentiation9. As one of the mechanisms by which cells recognize material properties, it has become clear that cells convert physical information from the surrounding environment into bioactive signals via cytoskeletal signaling pathways. When cells adhere to organ surfaces via the extracellular matrix (ECM), focal adhesion kinase (FAK) phosphorylates and activates the RhoA-ROCK signaling pathway, which consists of the Rho family protein RhoA and RhoA-binding kinase (ROCK). This promotes cross-linking of actin fibers, resulting in a broadly elongated, flattened morphology of the cells. On the other hand, inactivation of the RhoA-ROCK signaling pathway induces disassembly of actin fibers, resulting in an elongated spindle-like morphology. This RhoA-ROCK signaling is not only involved in cell morphology, but also functions as a molecular switch that regulates the differentiation lineage of mesenchymal stem cells, where its enhancement promotes osteoblastic differentiation of mesenchymal stem cells and induces differentiation into adipocytes in the presence of ROCK inhibitors10.
Therefore, we hypothesized that the differentiation lineage of stem cells could be controlled by utilizing the molecular mobility of polyrotaxane surfaces. In fact, our observation of the morphology of mesenchymal stem cells adhering to surfaces with different molecular mobility revealed the development of actin fibers and circular-radial adhesion morphology on surfaces with low molecular mobility, and elongated spindle-like adhesion morphology on surfaces with high molecular mobility11. The results suggest that the mesenchymal stem cell recognizes the molecular mobility of the polyrotaxane surface and alter its cytoskeleton and morphology. Next, we analyzed the activation of RhoA-ROCK in mesenchymal stem cells attached to each surface by real-time PCR and enzyme-linked immunosorbent assay, and the results showed that RhoA gene expression and ROCK enzyme activity were higher on surfaces with lower molecular mobility (Figure 2). This indicates that the molecular mobility of surfaces affects RhoA-ROCK signaling in mesenchymal stem cells. Furthermore, when mesenchymal stem cells were cultured in either osteoblastic or adipogenic differentiation induction medium, gene expression of Runx2, a marker protein for osteoblastic differentiation, was increased on surfaces with low molecular mobility, while gene expression of Pparg, a marker protein for adipogenic differentiation, was increased on surfaces with high molecular mobility. Functional evaluation of osteoblastic and adipogenic differentiation by alkaline phosphatase activity and Oil Red O staining was also consistent with those results. In other words, surfaces with low molecular mobility are useful for osteoblastic differentiation, while surfaces with high molecular mobility are useful for adipogenic differentiation. These results suggest that the molecular mobility of polyrotaxane may be a material parameter that defines stem cell differentiation lineages.
Recently, we have been further developing the effects of these molecularly mobile surfaces on various cell types, and have shown that they enhance hepatocyte function12, induce lumen formation in vascular endothelial cells13, enhance intercellular communication in co-cultures of vascular endothelial cells and mesenchymal stem cells14, switch inflammatory to anti-inflammatory properties of inflammatory cells15, enhance cell-cell adhesion in epithelial cells16, inhibit cancer cell migration and anti-cancer sensitivity17, and inhibit cellular senescence18. Largely because we, as researchers advocating biomaterials science, were blessed with many collaborators in the fields of medicine and dentistry, and gained insights from long years of steady research findings, we were able to clarify the importance of molecular mobility in biomaterial surface design.
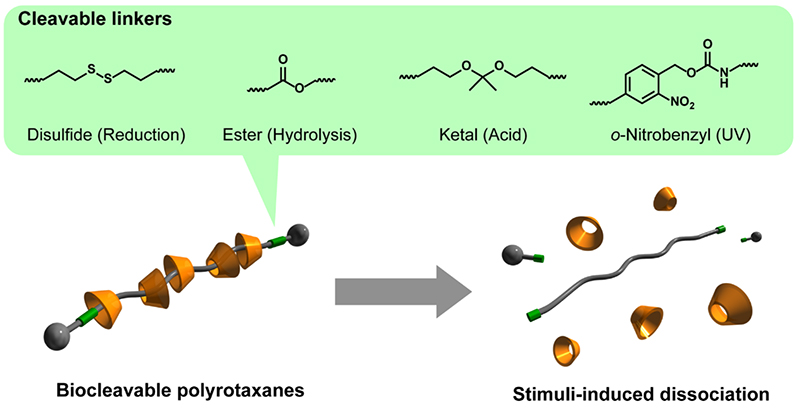
Many cyclic molecules in polyrotaxane are mechanically interlocked by bulky capping groups introduced at the ends of the axial polymer. However, when the capping groups are cleaved by chemical or physical stimuli, the polyrotaxane structure is quickly dissociated. Because of the supramolecular dissociation to such environmental changes and stimuli, polyrotaxane exhibits properties similar to biodegradable polymers. Such dissoiation properties can be imparted by incorporating cleavable linkages, such as reducible disulfide bonds or hydrolyzable ester bonds, between the axial polymer ends and the capping groups (Figure 3)19. This dissociation mechanism of polyrotaxane is essentially different from that of biodegradable polymers, such as aliphatic polyesters. Biodegradable polymers such as aliphatic polyesters require the cleavage of bonds in multiple locations for complete degradation. There are also concerns about toxicity and inflammation derived from the degradation products. On the other hand, biocleavable polyrotaxanes require only the cleavage of one cleavable bond to liberate all the threaded cyclic molecules, thus the time required for complete dissociation is overwhelmingly short and a rapid degradation response can be expected. Furthermore, since PEG and cyclodextrin, which are components of polyrotaxanes, are utilized in pharmaceuticals, etc., adverse effects derived from degradation products are expected to be minor.
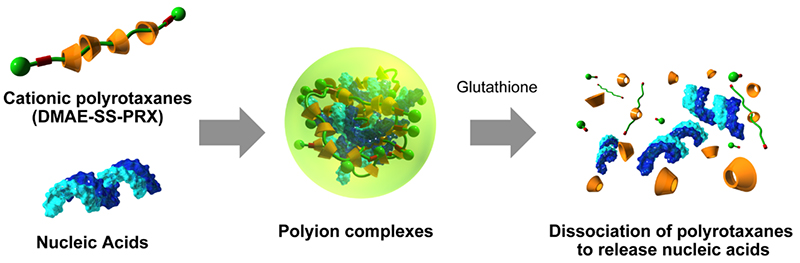
Intrigued by the possibility of applying biocleavable polyrotaxane, we are promoting research on drug delivery systems (DDS) for biomolecules. Polyrotaxanes modified with cationic N,N-dimethylaminoethyl (DMAE) groups at the α-cyclodextrin (α-CD) moiety (DMAE-PRX) can be used to form polyion complexes with anionic biomolecules such as nucleic acids and acidic proteins20-22. Forming an ion complex with DMAE-PRX results in a complex with positively charged surface, which improves interaction with negatively charged cell membranes and can significantly improve the efficiency of intracellular introduction of nucleic acids and acidic proteins, which are difficult for cells to take up. However, in terms of intracellular delivery, the function is the same as that of commercially available transfection reagents. It is necessary to release the cargos from the polyion complex is essential for emerging the bioactivity after internalization into the cell.
Since the concentration of glutathione, a biological reducing substance, is three orders of magnitude higher in the cytoplasm than in the extracellular environment, polyrotaxanes, which exhibits a dissociation in response to differences in reducing substance concentration, is expected to promote the dissociation and release of polyion complexes in the cell. Therefore, we synthesized cationic polyrotaxane with a disulfide bond in the axial polymer (DMAE-SS-PRX) that is specifically cleaved in a reductive intracellular environment (Figure 4)20. The polyion complex was prepared by mixing plasmid DNA (pDNA) encoding firefly luciferase gene and DMAE-SS-PRX. When introduced into HeLa cells, the DMAE-SS-PRX/pDNA complex showed the same level of gene expression as the poly(ethyleneimine)/pDNA complex, which has superior gene transfer efficiency. In addition, the DMAE-SS-PRX/pDNA complex showed gene expression levels about three orders of magnitude higher than those with pDNA complexes using non-degradable cationic polyrotaxane without disulfide bonds (DMAE-PRX), suggesting that the release of pDNA upon dissociation of polyrotaxane in the cell contributes to enhancing gene expression efficiency. We have also found that DMAE-SS-PRX is effective not only for the intracellular transduction of pDNA, but also for the intracellular delivery of siRNA and acidic proteins and the enhanced expression of their bioactivities21,22.
Derivatives of β-cyclodextrin (β-CD), which is composed of seven glucose molecules, have been used as excipients in pharmaceuticals. Additionally, because β-CD can form an inclusion complex with cholesterol, it is widely utilized as reagents for research purposes. Recently, β-CD has been shown to have therapeutic effects against diseases such as Alzheimer's disease and atherosclerosis, and its use as a medicine has attracted attention23,24. In particular, clinical trials of CD are underway as a treatment for Niemann-Pick type C disease (NPC disease), a type of lysosomal storage disorder25-27. The disease causes accumulation of cholesterol in cells throughout the body from birth, resulting in severe symptoms such as neurological regression, but no effective treatment has been established. Only after 2009 did it become clear that mouse models of NPC disease treated with 2-hydroxypropyl β-CD (HP-β-CD), a highly water-soluble β-CD derivative, show reduced tissue cholesterol accumulation, improved neurological function, and prolonged survival26,27. However, several problems have been pointed out in the use of HP-β-CD. For example, since HP-β-CD has a molecular weight of about 1500, it is excreted by the kidneys quickly after administration and consequently requires a very high dosage to obtain a sufficient therapeutic effect. Though HP-β-CD is a low-toxicity β-CD derivative, very high concentrations of HP-β-CD are administered in NPC disease and side effects such as tissue damage and hearing loss are of concern27,28. It is not easy to reduce the impact of the disability caused by β-CD derivatives because the same mechanism mediates both the side effects and therapeutic effect on NPC disease.
Here, the structure of polyrotaxane is such that the β-CD cavity is occupied by an axial polymer, so inclusion of cholesterol does not occur, which is thought to reduce the adverse effects of β-CD. In addition, if a large number of β-CDs can be released at the lysosomal site from dissociable polyrotaxane, it is expected to lead to more effective cholesterol excretion. Based on this hypothesis, we synthesized an acid-degradable polyrotaxane with inclusion complexes of β-CD. Since β-CD forms inclusion complexes with poly(propylene glycol) (PPG), we designed a β-CD/PEG-b-PPG-b-PEG triblock copolymer as an axial polymer and interlocked it with N-triphenylmethyl groups (Figure 5)29. In addition, a 2-(2-hydroxyethoxy)ethyl (HEE) group was modified at the β-CD moiety (HEE-PRX) to improve water solubility. The N-triphenylmethyl group, which is the capping group for the polyrotaxanes, is designed to be eliminated in acidic pH environments such as lysosomes to release the β-CD.
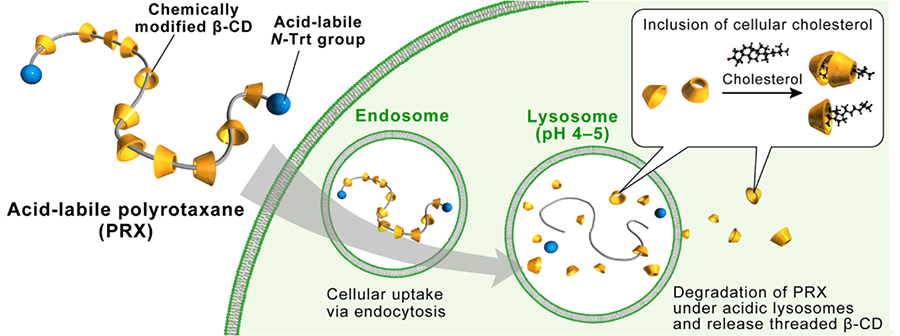
The effects of HEE-PRX on cytotoxicity and cholesterol accumulation were examined using skin fibroblasts derived from patients with NPC disease. HP-β-CD but not HEE-PRX caused cholesterol withdrawal from the cell membrane into the medium at high concentrations and reduced cell viability, indicating that the blocking of the β-CD cavity of the polyrotaxane structure prevented disruption of the β-CD molecule. A two-fold increase in cholesterol content was observed in NPC-derived cells compared to normal cells. HEE-PRX reduced intracellular cholesterol content to normal levels at about one-fiftieth the concentration of HP-β-CD. The dissociation of HEE-PRX in acidic pH lysosomes causing release of a large number of β-CDs locally is expected to promote cholesterol excretion.
Next, the cholesterol-lowering effect of polyrotaxane on NPC disease model mice was examined. NPC disease model mice at 8 weeks of age had about 10 times more cholesterol accumulated in their livers than normal mice. Weekly administration of HEE-PRX at 500 mg/kg to mouse models of NPC disease significantly suppressed tissue cholesterol accumulation. Administration of HP-β-CD at the same dose did not change tissue cholesterol content because the dose was significantly lower than previously reported (4,000 to 8,000 mg/kg)25,26. On the other hand, continued weekly administration of HEE-PRX at 500 mg/kg from the age of 3 weeks prolonged the survival of NPC disease model mice by 2 to 3 weeks. The same dose of HP-β-CD did not significantly change the survival period, indicating that HEE-PRX has a therapeutic effect even at low doses that do not produce a therapeutic effect with HP-β-CD30.
Thus, intracellularly dissociable polyrotaxane is expected to be an effective medicine because it avoids the adverse effects caused by β-CD derivatives and shows a therapeutic effect on NPC disease by suppressing cholesterol accumulation even at low doses. In other words, the new drug with the dissociable properties of polyrotaxane has clearly demonstrated effects that cannot be achieved by the aforementioned β-CD derivatives alone, and has certainly opened up a new area of research that could expedite further development of clinical treatment for intractable metabolic diseases. The lowering of intracellular cholesterol by HEE-PRX has been shown to be effective in the treatment of not only NPC disease but also lifestyle-related diseases such as atherosclerosis31. Furthermore, because HEE-PRX suppresses inflammatory responses31 and osteoclast differentiation32, its use in the treatment of various diseases is expected.
Both molecular mobility and degradation responsiveness are at the core of the biomaterial functional design that actively utilizes the unique characteristics of polyrotaxane with assembly via mechanical linkage. Thirty years have passed since we started our research of these properties in 1993. During these periods, we have achieved many research milestones by taking advantage of molecular mobility and degradation responsiveness, but it is also true that we are still only half way to reaching the goal of a practical science of biomaterials. It is historically clear that scientific paradigm shifts are brought about in three stages: the philosophy, revolutionary, and completion. The polyrotaxane design of the authors represents somewhere between the philosophy and revolutionary stage, and further academic collaboration and cooperation with industry are essential to reach the completion. We think that the biomaterials science is for someone who come later, and leave a legacy for others to follow, and we have always conducted our research with this in mind. We sincerely hope that the next generation of researchers will be able to surmount the hurdles that we have not been able to overcome over the course of half our lives, and that as a result, this work on polyrotaxane will be strongly contributing to reaching the completion in the biomaterials science.
The work presented here is the result of collaborative research conducted over 30 years from 1993 to 2023 with many professors and students, including Professor Emeritus Kazuhiko Ishihara of the University of Tokyo, Professor Tetsuji Yamaoka, former Director of Biomedical Engineering at the National Cardiovascular Research Center, Professor Emeritus Hideyoshi Harashima of Hokkaido University, Professor Atsushi Maruyama of the Tokyo Institute of Technology, Associate Professor Tooru Ooya, Kobe University; Professor Yumiko Oishi, Nippon Medical School; Professor Akio Kishida, Tokyo Medical and Dental University; Professor Sachiko Iseki, Professor Tetsuya Yoda, Professor Takanori Iwata, Professor Takashi Ono; Former Assistant Professor Ji-Hun Seo (currently Associate Professor at Korea University); Former Assistant Professor Yoshinori Arisaka; Dr. Kei Nishida (currently Assistant Professor at Tokyo Institute of Technology); Dr. Asato Tonegawa, Dr. Dae-Hoon Lee, Dr. Ruriko Sekiya-Aoyama, Dr. Tae Woong Kang, Dr. Moe Ohashi (currently PMDA), Dr. Shunyao Zhang, Dr. Izumi Fukumoto, Dr. Hideto Matsui, Dr. Kai Shibaguchi, Dr. Masahiko Terauchi, Dr. Takasuke Inada, Dr. Katsuya Hyodo, Dr. Hiroki Masuda, Dr. Hongfei Zhu, Dr. Arun K. Rajendan, Dr. Masahiro Hakariya, Dr. Ryo Mikami, and Dr. Yuka Tanaka-Takemura. We would like to express our sincere gratitude to them.