
Toshiyuki Kida
Department of Applied Chemistry, Graduate School of Engineering, Osaka University. Professor
Toshiyuki Kida received his PhD in 1998 from Osaka University under the supervision of Professor I. Ikeda. He became an Assistant Professor in the Department of Applied Chemistry, Faculty of Engineering, Osaka University in 1991 before receiving his PhD degree. He was promoted to a Lecturer at the Graduate School of Engineering, Osaka University in 2004, and then to an Associate Professor at Graduate School of Engineering, Osaka University in 2006. In 2016, he became a full Professor in the Graduate School of Engineering, Osaka University. He worked with Professor Bradley D. Smith, University of Notre Dame (USA) in 1999-2000.
Cyclodextrins (CDs) are a class of cyclic oligosaccharides consisting of several α-(1,4)-linked D-glucopyranose units (Figure 1A). CDs composed of 6, 7, and 8 glucosidic units are called α-, β-, and γ-CDs, respectively, and have been used extensively. They have a sub-nanometer-sized cavity that can accommodate guest molecules of the appropriate size and shape. The inclusion ability of CD has been academically studied as a catalyst, sensor, etc., and has been widely utilized industrially in foods, cosmetics, and pharmaceuticals1-4. On the other hand, CDs can regularly assemble intermolecularly through hydrogen bonding between hydroxyl groups on the upper and lower rims of their doughnut-shaped rings or through host-guest molecular interactions. Recently, much attention has been paid in the fields of supramolecular chemistry and materials science to studies on nano- and microstructures formed by the assembly of CDs5,6. This article reviews the preparation of supramolecular structures by using the regular assembly of CD molecules, and the morphological control of supramolecular structures.
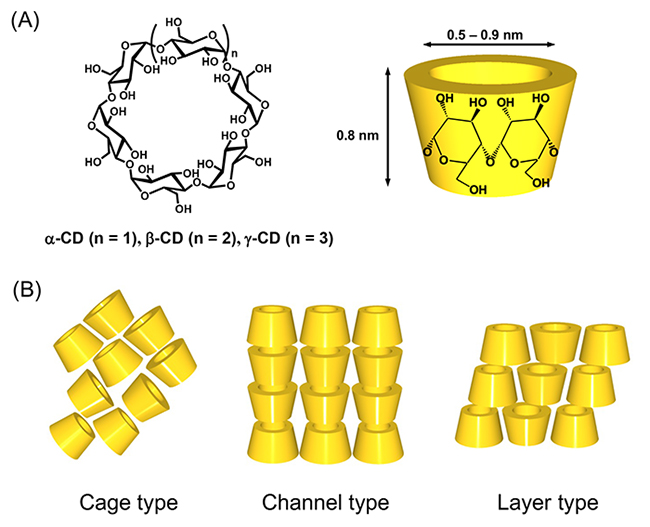
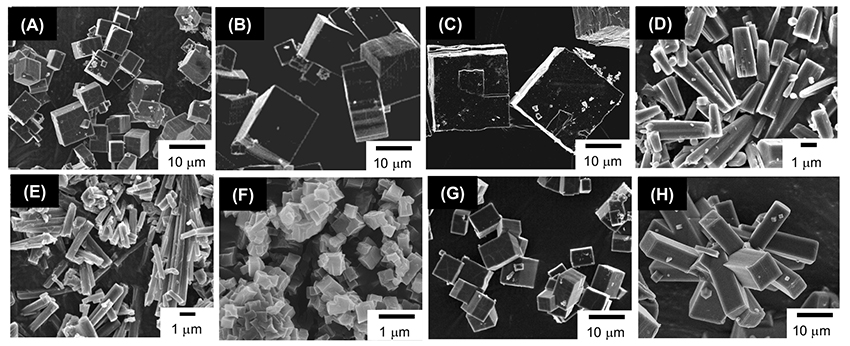
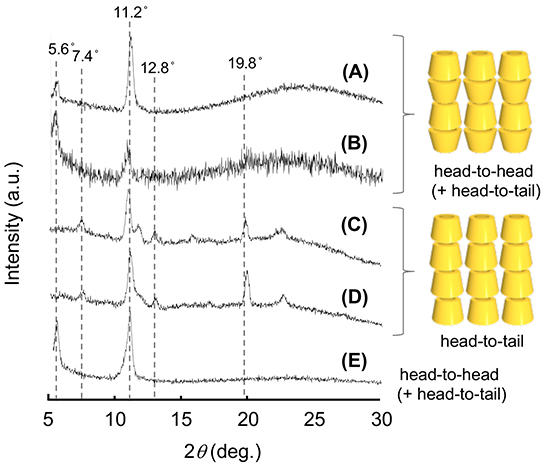
In crystals, CDs adopt three types of assembly modes known as cage, channel, and layer types (Figure 1B)7. In the channel-type assembly, CD molecules are stacked linearly in a head-to-head or head-to-tail fashion through hydrogen bonding between the hydroxyl groups of neighboring CD molecules to form a columnar structure. The channel-type assembly has been prepared by forming inclusion complexes between CDs and guest molecules, especially between CDs and polymeric guest molecules. A facile method for the preparation of channel-type assemblies from CD molecules in the absence of any guest molecules was developed by Tonelli et al8. They succeeded in the preparation of channel-type assemblies of α- and γ-CDs by dropping an aqueous CD solution into chloroform and acetone, respectively. The authors prepared channel-type assemblies of γ-CD (γ-CDchannel) using Tonelli’s method and observed the formation of unique cubic microstructures by scanning electron microscopy (SEM) (Figure 2A)9. The authors also succeeded in preparing CD structures with various morphologies by varying the fabrication conditions, such as the γ-CD concentration in the aqueous solution (Figure 2)10. γ-CD microcubes with an average edge length of 7 μm (Figure 2A) are fabricated by dropping a saturated aqueous γ-CD solution into a poor solvent acetone at ambient temperature. The size of the γ-CD cubes increased with decreasing γ-CD concentration in the aqueous solution (Figure 2B, C). For instance, the size of the γ-CD cubes grew about four times larger than the original microcube when we decreased the γ-CD concentration to one-hundredth of its original concentration (Figure 2C). We also examined the effects of guest inclusion into the γ-CD cavity on the morphology of the resulting γ-CD microstructure. Potassium iodide (KI) and sodium perchlorate (NaClO4) were chosen as guests. When an aqueous γ-CD solution (0.17 M) including 1 equiv. of KI (0.17 M) was dropped into 2-propanol, nanometer-sized cubes (average edge length 300 nm) were formed (Figure 2F). In the presence of 0.1-0.5 equiv. of KI, rod-like microstructures were produced (Figure 2D, E). These microrods became thinner as the amount of KI increased from 0.1 to 0.5 equiv. On the other hand, in the presence of NaClO4, different γ-CD microstructures tended to form. γ-CD microcubes resulted when the amount of added NaClO4 was less than 0.9 equiv. (Figure 2G), and microrectangular particles resulted when it was more than 1 equiv. (Figure 2H). These results showed that the morphology of the γ-CD structures can be controlled by the type and amount of guest anions incorporated into the γ-CD cavities. The X-ray diffraction (XRD) patterns of these microcubes, which all showed a strong peak at around 7.5° characteristic of the channel structure of γ-CD, clearly showed that they were composed of channel-type assemblies of γ-CD.
1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) has been used to dissolve peptides and polymers that are insoluble in common organic solvents11,12. The authors found that CDs have a high solubility in HFIP. The solubility of α-, β-, and γ-CD in 100 mL of water at room temperature is 14.5 g, 1.8 g, and 23.2 g, respectively2, whereas the solubility of each CD in 100 mL of HFIP at room temperature is 25 g, 34 g, and 25 g, respectively13, showing that the solubility of all CDs in HFIP exceeds that of those in water. In particular, the solubility of β-CD is much higher in HFIP than in water. NMR analysis revealed an HFIP molecule inside the cavity of β-CD, and this inclusion is thought to be related to the high solubility of β-CD in HFIP.
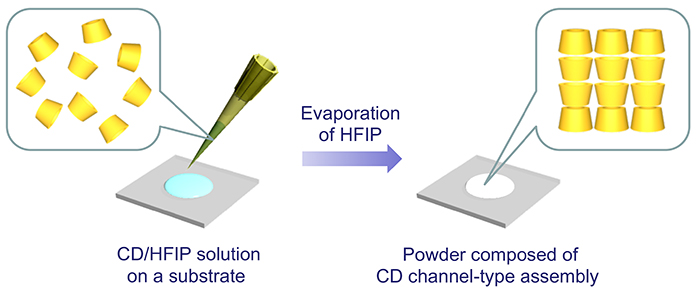
In addition, HFIP has a relatively low boiling point and can be evaporatively removed from CD solutions under mild conditions. The authors found that a crystalline solid composed of channel-type assemblies of each CD can be formed by evaporating HFIP from an HFIP solution (0.1 mol/L) of α- or γ-CD dropped on a substrate at room temperature (Figure 3). On the other hand, a mixture of cage-type and channel-type assemblies was obtained from HFIP solution of β-CD. All of these CDs formed cage-type assemblies before treatment. Thus, in the cases of α- and γ-CD, a complete transformation of the assembly mode from cage-type to channel-type occurred, but in the case of β-CD, unconverted cage assemblies remained. HFIP molecules inside the β-CD cavity are thought to inhibit the transformation.
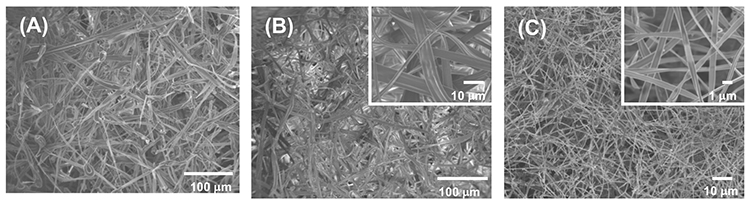
HFIP solutions of α-, β-, and γ-CD were electrospun (voltage: 25 kV, nozzle-accumulator distance: 10 cm, speed: 0.16 mL/min) into microfibers with widths of 8.3 ± 3.4 μm, 5.3 ± 2.0 μm, and 0.75 ± 0.21 μm, respectively (Figure 4). No significant peaks were observed in the XRD patterns of these microfibers, indicating that the microfibers consist of amorphous CD assemblies. The CD/HFIP solution formed microfibers at a much lower concentration (13 wt% or less) than previously reported for CD microfiber preparations that required a high concentration of CD solution of 60 wt% or more14-16. These results reveal that the CD/HFIP solution method is very effective for microfiber formation.
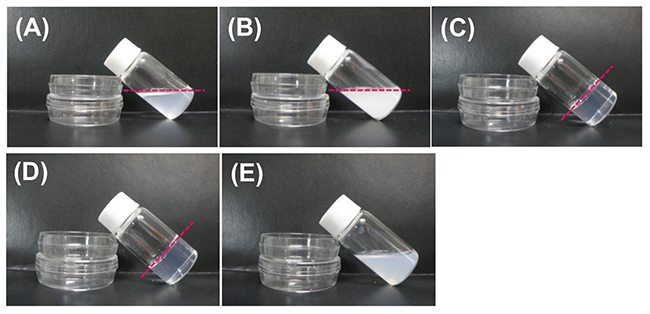
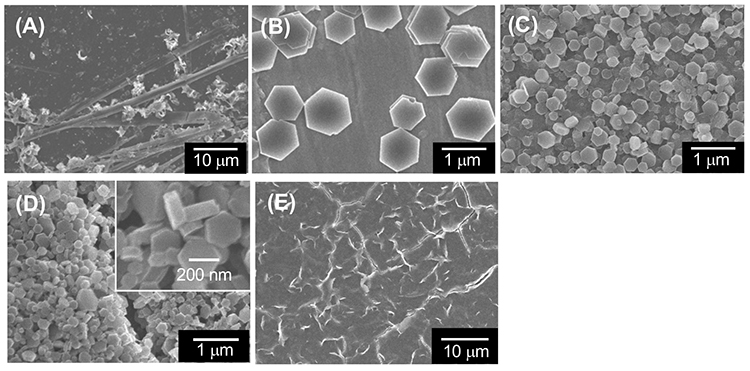
Dropping an HFIP solution of α-CD (0.5 mL, 24 mg/mL) into a five-fold volume of the poor solvent 2-butanol (2.5 mL), stirring the mixture for 3 h, and allowing it to stand for 3 days at ambient temperature, transformed the mixture into an organogel (Figure 5C)17. On the other hand, when 1- and 2-propanol were used as poor solvents, organogel formation was not observed (Figure 5A, E). SEM images of the xerogels and the solids which were obtained by drying the organogels (from 2-butanol) and the non-gelated suspensions (from 1- and 2-propanol), respectively, showed the morphology of supramolecular structures existing in the organogels and suspensions. Interestingly, SEM images of the xerogels indicate that these organogels are composed of nanometer-sized hexagonal plate-like structures (Figure 6C), while drying of non-gelated 1- and 2-propanol suspensions affords tape-like structures and micrometer-sized hexagonal plates, respectively (Figure 6A, B). XRD measurement shows that the xerogels are composed of head-to-tail channel assemblies of α-CD, which are hexagonally aligned in the horizontal direction. On the other hand, the solids obtained from non-gelated suspensions are composed of a channel-type α-CD assembly with a head-to-head orientation or a mixture of head-to-head and head-to-tail orientations, which are not regularly aligned in the horizontal direction. Considering these results, the difference in the assembly mode of α-CD molecules appears to correlate with the morphology of the resultant supramolecular α-CD structure, demonstrating that the morphology affects organogel formation. We also examined the effect of the chirality of the poor solvent on organogel formation. When (S)-2-butanol is used as a poor solvent, organogels form after allowing the mixture to stand for 36 h (Figure 5D). On the other hand, when (R)-2-butanol is used, no organogel forms in the mixture even after allowing it to stand for 48 h (Figure 5E). These results clearly show that the solvent chirality-selective organogelation is attained via combining α-CD/HFIP solution with 2-butanol. In the SEM images of xerogels from the (S)-2-butanol gel, hexagonal nanoplates are observed (Figure 6D), morphologically similar to the nanoplate structures of xerogels from the rac-2-butanol gel (Figure 6C). On the contrary, in the SEM images of the solids obtained by drying the non-gelated suspensions of (R)-2-butanol, no regular nano- and microstructures are observed (Figure 6E). XRD patterns of these solids show that the xerogels from (S)-2-butanol are composed of channel-type α-CD assemblies with a head-to-tail α-CD orientation, which are hexagonally aligned in the horizontal direction (Figure 7D). On the other hand, the solids from the non-gelated suspensions are composed of a channel-type α-CD assembly with a head-to-head orientation or with a mixture of head-to-head and head-to-tail orientations. These results show that, in the case of chiral 2-butanol, the formation of three-dimensional hexagonal nanoplates composed of a head-to-tail α-CD channel assembly correlates closely with organogel formation. The time required for organogel formation decreased as the ratio of S- to R-isomer of 2-butanol increased. This suggests that the inclusion complex formed between α-CD and (S)-2-butanol is involved in organogel formation.
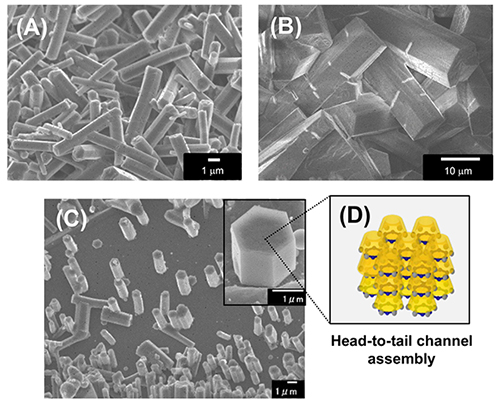
A methylated derivative of α-CD at the 2-O position (2-Me-α-CD) was used as a building block of hexagonal rod-like microstructures composed of head-to-tail channel assemblies18. These supramolecular structures were prepared by dropping a methanol solution of 2-Me-α-CD (0.5 mL, 10 mg/mL) into various poor solvents such as cyclohexane and benzene (2.5 mL), then stirring the solution, allowing it to stand at room temperature, filtering the resulting precipitate, and drying the precipitate in a current of N2 for XRD and SEM measurements. When cyclohexane and benzene were used as poor solvents, the precipitates formed hexagonal microrods (Figure 8A , B). The XRD pattern of the microrods confirms the formation of a horizontal hexagonal close-packed arrangement of head-to-tail channel assemblies.
The X-ray single crystal structure of the 2-Me-α-CD-benzene inclusion complex showed that one benzene molecule was included near the wider rim of 2-Me-α-CD (Figure 9), and the axis of the benzene molecule was perpendicular to the main axis of the CD cavity. 2-Me-α-CD molecules adopted a head-to-tail channel–type arrangement via hydrogen bonding between the 3-OH group and the CH2 group at the C-6 position with an O···H distance of 2.73 Å.
Furthermore, by dropping a mixture of 2-Me-α-CD/methanol solution and benzene onto a highly oriented graphite (HOPG) substrate and drying it, hexagonal rod-like microstructures composed of the 2-Me-α-CD-benzene inclusion complex are formed via epitaxial growth on the HOPG substrate (Figure 8C , D). Benzene molecules incorporated into the cavity of 2-Me-α-CD bind to the graphene surface through π-π interactions, and this binding triggers the epitaxial growth of a 2-Me-α-CD–benzene inclusion complex crystal on the HOPG substrate.
CDs form channel-type assemblies mainly through hydrogen bonding between the hydroxyl groups on the upper and lower rims of the CD rings. Moreover, these assemblies three-dimensionally aggregate to form supramolecular structures with various morphologies. Channel-type assemblies of α-CD and γ-CD mainly form hexagonal and tetragonal structures, respectively. It was revealed that the morphology of the supramolecular structure is largely affected by the ring size of CDs as building blocks. We also found that the morphology of the supramolecular structure changes depending on the type of guest molecules included in the CD cavity. The morphology of the supramolecular structure can be expected to control functions such as ability to form organogels and host-guest inclusion complexes. In the future, more studies on nano- and microstructures formed by regular assembly of CD molecules, which are environmentally compatible functional materials, will be carried out in a variety of fields, including supramolecular chemistry, separation chemistry, and materials science.
The author would like to express his sincere thanks to Professor Emeritus Isao Ikeda and Professor Emeritus Mitsuru Akashi of Osaka University for their guidance and suggestions. I would also like to express my sincere gratitude to all the co-workers who gave me useful advice, and to the students who produced various interesting experimental results.