
Takashi Hayashita
Professor Takashi Hayashita received his PhD from Kyushu University in 1985. After working as a research associate in Kanagawa University, he spent several years at Texas Tech University as a postdoctoral fellow. He joined the Faculty of Science and Technology at Sophia University in 2005 as Professor following his teaching experience at Saga University and Tohoku University as Associate Professor. Since 2010 he has been serving as the Dean of Faculty of Science and Technology as well as the Chair of Graduate School of Science and Engineering, and in 2014 he became the 15th President of Sophia University. He served as the President of the Japan Society for Analytical Chemistry (2021-2022), the Japan Society of Cyclodextrins (2020-2021), the Japan Society of Ion Exchange (2020-2021), and the Association for Research of Host-Guest and Supramolecular Chemistry (2020-2023).
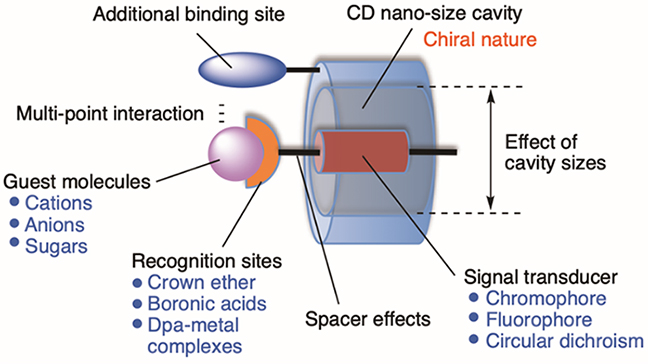
Supramolecules based on non-covalent interactions can rearrange in response to changes in the environment and change their function as complexes1. We have been developing supramolecular analytical reagents that focus on the dynamic molecular recognition function of these supramolecules. In particular, we have developed molecular recognition systems based on the inclusion ability of cyclodextrins (CDs: a hexamer is called α-CD, a heptamer is called β-CD, and an octamer is called γ-CD). The inclusion ability of CDs is, in turn, based on the structural changes in the CD cavity of molecular recognition probes, such as metal ion recognition, phosphate derivative recognition, and sugar recognition, as well as the creation of supramolecular chirality built on the chirality of CD cavities derived from natural products (Fig. 1)2,3. We have also succeeded in developing spherical ultrasmall CD nanogels with uniform CD orientation by using cationic amphiphilic compounds as emulsifiers, and have revealed their excellent inclusion capabilities4,5. In this review, the development of these CD-based supramolecular analytical reagents is summarized.
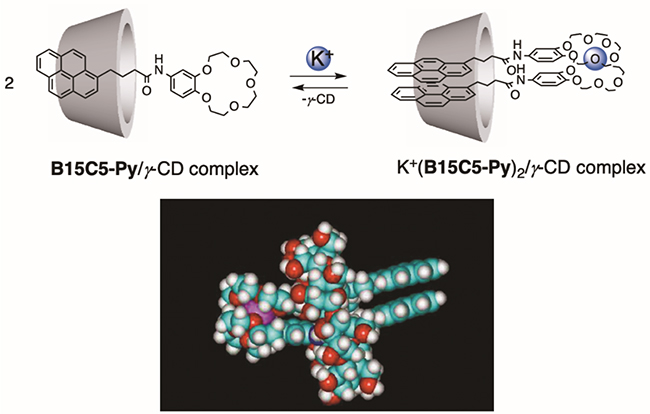
In 1977, Takagi et al. reported the world's first crown ether-type colorimetric reagent for use as an analytical alkali metal ion recognition system6. However, because the interaction between alkali metal ions and crown ethers is weak in water, this colorimetric response had to be carried out in an organic solvent such as chloroform. We have focused on the ability of CDs to dissolve hydrophobic compounds in water, and demonstrated an excellent response function to alkali metal ions in water using a complex of crown ether type fluorescent probe B15C5-Py and γ-CD7. Benzo-15-crown-5-ether forms a sandwich-type complex with K+ ions. We reported that when 2+ possessing the largest cavity in water is used, two molecules of a fluorescent probe enter the cavity, and the selective dimer emission of the pyrene fluorophore appears for K+ ions (Fig. 2)8. Similarly, in a fluorescent probe with a podand-type recognition site, selective dimer emission appears for Pb2+ ions9. In this way, the cavity of γ-CD functions as a nano-space molecular flask, and it has become clear that the metal ion-dependent dimer formation within the cavity is extremely useful for the design of novel supramolecular analytical reagents.
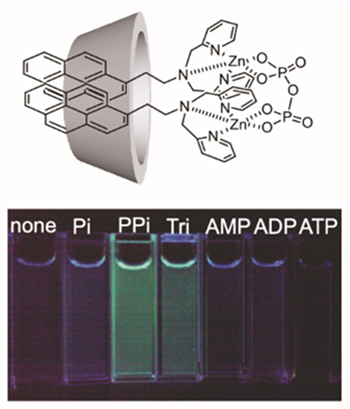
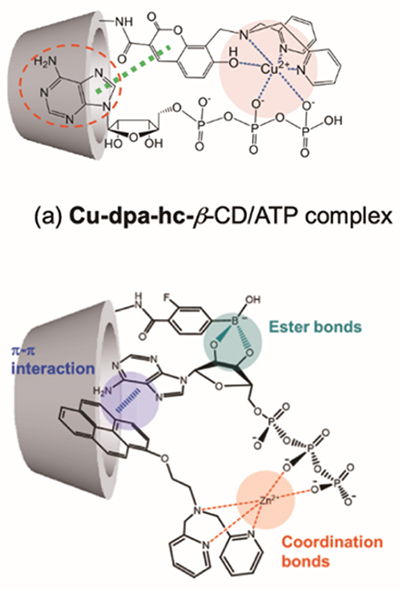
A fluorescent probe in which the molecular recognition site has been changed from crown ether to a metal complex of dipicolylamine (dpa) shows dimer emission in γ-CD in response to phosphate derivatives. The CD complex responds to pyrophosphate and triphosphate ions but not adenosine triphosphate (ATP), and the fluorescent color changes due to dimer emission (Fig. 3)9. However, in order to distinguish between highly bioactive ions such as ATP and inorganic phosphate ions, ingenuity in molecular design is required. We have succeeded in developing an ATP-selective fluorescent reagent by chemically modifying the secondary hydroxyl group of β-CD with a dpa-Cu2+ complex, Cu-dpa-hc-β-CD, in which the adenine site of ATP is recognized in the cavity of β-CD (Fig. 4a)10. The binding constant for ATP is 3.3 x 103 M-1. On the other hand, when a modified CD (3-FPB-γ-CD) is prepared by chemically modifying the secondary hydroxyl group of γ-CD with phenylboronic acid having a fluorine unit at the 3-position (3-FPB), and combining it with Zn-dpa-OC2Py probes of various spacer lengths, the Zn-dpa-OC2Py/3-FPB-γ-CD complex is found to show excellent ATP selectivity, with a binding constant of 6.2 x 106 M-1, which is nearly 1000 times higher than that of Cu-dpa-azo-β-CD (Fig. 4b)11. Interestingly, the Zn-dpa-OC6Py/3-FPB-γ-CD complex with a longer spacer length completely lost ATP selectivity. This indicates that the spatial arrangement of the CD complex based on multi-point recognition of ATP plays an important role in increasing the selective binding ability. This is a feature that can only be seen in supramolecular complexes.
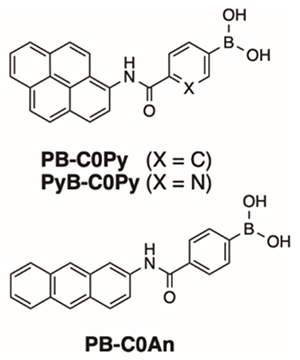
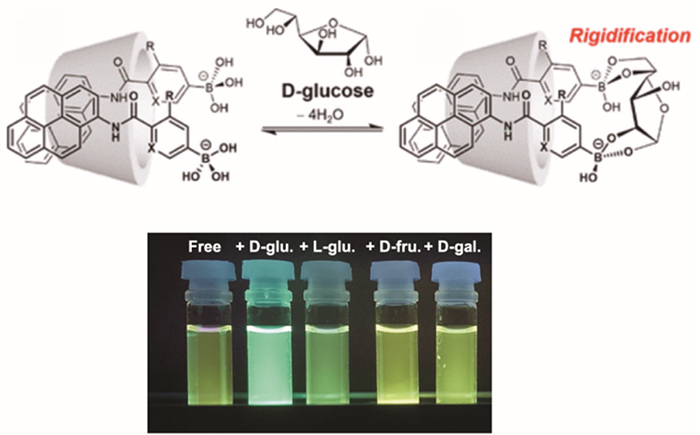
Phenylboronic acid is known to form stable esters with the cis-diol moiety of sugars in water. Among monosaccharides, the phenylboronic acid shows high selectivity for fructose, but glucose can be esterified with phenylboronic acid at two positions in the molecule, and hence the glucose selectivity can be obtained by appropriately positioning the phenylboronic acid12. We have found that the simple phenylboronic acid-based azo probe BA-Azo-Ph selectively forms a dimer with glucose in the cavity of γ-CD (Fig. 5)13. , PB-C0Py, the phenylboronic acid-based fluorescent probe in which a pyrene fluorophore and a phenylboronic acid are directly linked by the amide bond(Fig. 6), recognizes glucose in the cavity of γ-CD and forms a dimer showing glucose-selective dimer emission. Interestingly, when the pyrene fluorophore is replaced by anthracene with a slimmer skeleton(Fig. 6), the selectivity changes from glucose to galactose14. This result indicates that the spatial arrangement of the two fluorescent probes in the γ-CD cavity affects the selectivity. In the complex of 3-FPB-β-CD chemically modified by addition of 3-FPB with the anthracene-type probe, two-point recognition occurs between the phenylboronic acid on the CD side and the boronic acid-type fluorescent probe, and glucose selectivity similar to that of the dimer can be obtained15. The fluorine unit introduced into phenylboronic acid is electron-withdrawing, which reduces the pKa of phenylboronic acid, enabling glucose recognition under physiological pH conditions (neutral pH). For the same purpose, we also designed a fluorescent probe C0-APyB using pyridylboronic acid, and interestingly we found that the C0-APyB/γ-CD complex can distinguish between the enantiomers D-glucose and L-glucose. This chiral recognition function is based on the chiral environment of the CD cavity. By inserting two molecules of a rigid pyrene-type probe, a highly specific chiral recognition function is realized (Fig. 7)16. This fluorescent probe also has promising applications as circularly polarized luminescence materials.

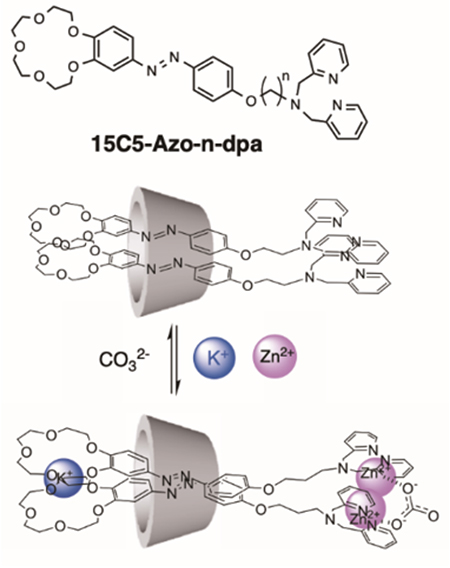
As mentioned in the previous section, natural CDs dissolve in water and provide a chiral hydrophobic field that encapsulates molecules in their cavities. We have reported that the supramolecular complex, consisting of a ditopic azo probe 15C5-Azo-n-dpa having two molecular recognition sites with γ-CD that can encapsulate two molecules of this probe in water, exhibits a unique supramolecular chirality only in the presence of multiple guest molecules and ions (Fig. 8)17. This supramolecular complex induces a clockwise twisting structure of the two ditopic probes in the γ-CD cavity and exhibits responsive ability only in the presence of a K+ ion that forms a sandwich complex with the crown ether moiety, a Zn2+ ion that forms a complex with the dpa moiety, and a CO32- ion that bridges the two dpa-Zn2+ complexes. Therefore, when one of the components inducing the twisted structure is removed from this supramolecular complex, the complex selectively responds to the removed element ion and exhibits changes in circular dichroism spectrum and UV-visible absorption spectrum based on the twisted structure of the azo moieties. More interestingly, changing the alkyl spacer from an ethylene chain (n = 2) to a butylene chain (n = 4) dramatically changes the metal ion response selectivity from Zn2+ to Cu2+ ions18. Although both are ditopic probes with the same molecular recognition sites, no system in which the response selectivity changes only with the spacer length has been reported in the molecular recognition reactions to date. These results also demonstrate the importance of spatial arrangement based on multiple guest ion recognition in the nano-space inclusion cavity of γ-CD, and are certainly a unique feature of supramolecular complexes.
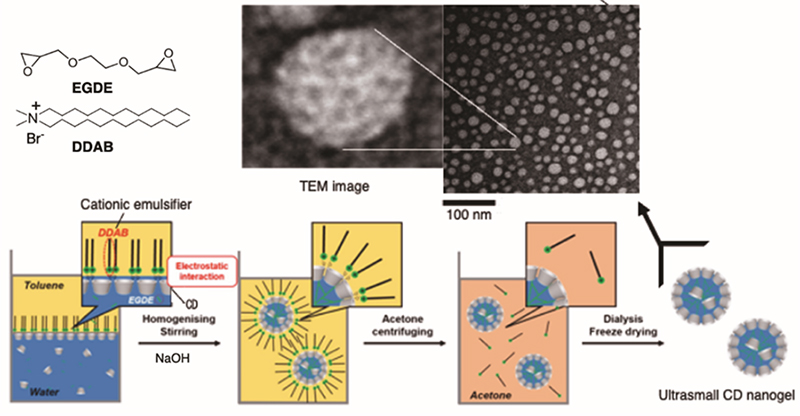
Since CD has multiple primary and secondary hydroxyl groups in the molecule, water-insoluble CD gels can be obtained by reacting CDs with a crosslinker such as ethylene glycol diglycidyl ether (EGDE) under alkaline conditions. By incorporating a naphthaleneboronic acid-type fluorescent probe that emits fluorescence upon binding to sugar into the CD gel, we have succeeded in developing a CD gel sensor that concentrates trace amounts of fructose within the CD gel and shows a fluorescent response19, as well as a glucose-selective CD gel adsorbent based on the molecular template20. However, the attractive feature of CDs is that they enable dissolution of water-insoluble probes and drugs in water, and we have devised a method for preparing CD gels in nano-sized emulsion droplets using cationic dilauryldimethylammonium bromide (DDAB) as an emulsifier. The interface of the emulsion droplets of DDAB in toluene is positively charged, and when reacted with EGDE under alkaline conditions using NaOH, the secondary hydroxyl groups (pKa = 12.1) on the wide-rim side of CDs are dissociated and oriented at the DDAB interface, allowing CD gel formation to take place. As a result, we succeeded in developing uniform spherical ultrasmall CD nanogels of about 5 nm in size in which the secondary hydroxyl groups of CDs are facing outward (Fig. 9)4. Langmuir-type binding analysis reveals that the ultrasmall CD nanogels have superior encapsulation properties compared to monomeric CDs4. In the future, the ultrasmall CD nanogels are expected to be utilized in various fields such as in vivo drug delivery and bioimaging systems5,21.
To develop completely novel analytical reagents, a new methodology different from conventional synthetic chemical approaches is necessary. As this methodology, we have created the design of supramolecular analytical reagents that are based on the nano-space inclusion cavity of CDs. The supramolecules bound by non-covalent interactions can rearrange in response to changes in the environment and vary their functions as complexes. The diverse molecular recognition functions of nano-space cavities in various CD complexes and ultrasmall CD nanogels described in this review are certainly a unique feature of supramolecular analytical reagents.
I would like to thank my supervisor at Tohoku University, Professor Emeritus N. Teramae (Tohoku University), my co-researchers Dr. S. Nishizawa (currently a professor at Tohoku University), and Dr. A. Yamauchi (currently an assistant professor at Nara Medical University). Also I would like to take this opportunity to express my gratitude for their research support to Associate Professor A. Endo (who passed away in December 2018) and Professor T. Hashimoto, both faculty members at Sophia University, Dr. Y. Tsuchido (currently a lecturer at Waseda University), Dr. S. Fujiwara (currently an assistant professor at Kanagawa University), Dr. Y. Suzuki (currently an assistant professor at Saitama University), and the nearly 200 graduates of our Analytical Chemistry Research Group in the Department of Materials and Life Sciences, Sophia University. Furthermore, I would like to express my sincere gratitude to the Japan Society for the Promotion of Science for providing research grants, including a Grants-in-Aid for Scientific Research (A) (26248038), Grants-in-Aid for Scientific Research (B) (20H02772; 22350039; 18350043), a Grant-in-Aid for Scientific Research (C) (16550066), and Challenging Exploratory Research grants (24655069; 21655030).