
Hatsuo Yamamura
Professor at Graduate School of Engineering, Nagoya Institute of Technology
He graduated from the Faculty of Pharmaceutical Sciences, Kyushu University in 1984 and obtained his M.D. from the Graduate School of Pharmaceutical Sciences of Kyushu University in 1986. In the same year, he was employed by Asahi Breweries, Ltd. He was appointed assistant researcher at the Faculty of Pharmaceutical Sciences, Fukuyama University in 1989 and at Nagoya Institute of Technology, Faculty of Engineering in 1991. In the same year, he received his Ph.D. from Nagasaki University. In 2001, he was promoted to associate professor at Nagoya Institute of Technology, and then professor in 2006. He won the SCDJ Award from The Society of Cyclodextrins, Japan in 2023.
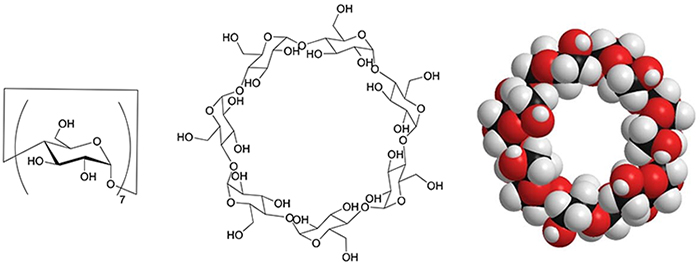
Cyclodextrins (CDs) are cyclic oligosaccharide molecules with a size of 1 nm, consisting of glucose units (Fig. 1). This molecule has the property of inclusion, i.e., it allows a variety of molecules to enter the cavity of its ring structure. It not only is the subject of academic study but also is employed extensively in practice in a number of industries, including food, cosmetics and pharmaceuticals. Another salient feature is that these molecules have a number of hydroxy groups. In general, it is possible to convert hydroxy groups into various functional groups through chemical modification. This allows the synthesis of CDs with novel structures and capabilities. Here, we describe the work to create antibacterial CD derivatives by introduction of cationic and hydrophobic groups to disrupt bacterial cell membranes.
“COVID-19 has demonstrated the importance of strengthening preparedness for global health threats, including the ‘silent pandemic’ of antimicrobial resistance (AMR). AMR is already having significant impacts on our economies and health systems, leading to an estimated 700,000 deaths globally from drug-resistant infections annually.
Despite this AMR threat, different factors including market failure contribute to the lack of development of new antibiotics, with no new class of antibiotic coming to market for more than three decades”
(G7 Finance Ministers’ Statement on Actions to Support Antibiotic Development, 2021).
Antimicrobial resistance is a significant threat to humanity and a topic continually discussed at the Group of Seven (G7) summit meetings. Furthermore, the antimicrobial resistance situation in Japan has been developing gradually at an uninterrupted pace. In 2019, it was for the first time reported that 8,000 deaths per year were caused by two distinct pathogens, namely, methicillin-resistant Staphylococcus aureus (MRSA) (Fig. 2) and fluoroquinolone-resistant Escherichia coli1. If the worst-case scenario materializes, it is estimated that by 2050 up to 10 million people could die per year on a global scale2.
The majority of antimicrobial agents exert their efficacy by inhibiting the activity of proteins that are essential for bacterial survival. In response, bacteria can undergo genetic mutation to alter their proteins and thereby acquire drug resistance. In order to address the issue of drug resistance, antimicrobial peptides have emerged as a promising area of research in recent years3. They disrupt bacterial membranes and can rapidly eliminate bacteria. Furthermore, they are efficacious against bacteria that have developed resistance to conventional antimicrobials, as their mechanism of action differs from that of conventional antimicrobials. It is metabolically challenging for bacteria to alter bacterial membranes, thereby reducing the likelihood of developing new resistance. However, many antimicrobial peptides cause side-effects such as hemolysis because they disrupt the membranes of animal cells as well as bacterial membranes. Furthermore, improving the peptide structure in order to reduce toxicity is not a straightforward task. Despite the large amount of academic research devoted to this topic, only a few antimicrobial peptides have been put to practical use as a therapeutic agent.
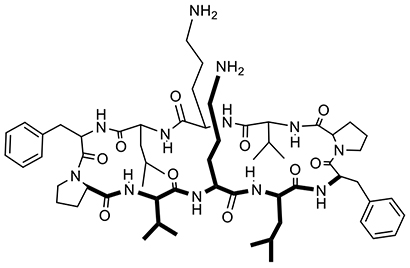
We have investigated the synthesis of derivatives through chemical modification of CDs. This has included the introduction of functional groups regiospecifically on CDs for molecular recognition including chiral recognition4-6. Meanwhile, we also examined antimicrobial peptides7,8. The antimicrobial peptide gramicidin S (Fig. 3) has a β-sheet structure, and contains cationic amino groups and hydrophobic alkyl groups. These groups cooperatively interact with lipid phosphate groups on the surface and within the lipid bilayer of bacterial membranes, resulting in membrane damage. We were inspired by the rational structure and skillful action of natural antimicrobial peptides. This led us to hypothesize that CDs, which are similar in size to peptides, could develop antimicrobial properties if equipped with the appropriate functional groups. Consequently, we initiated studies of CD derivatives as biomimetics of antimicrobial peptides.
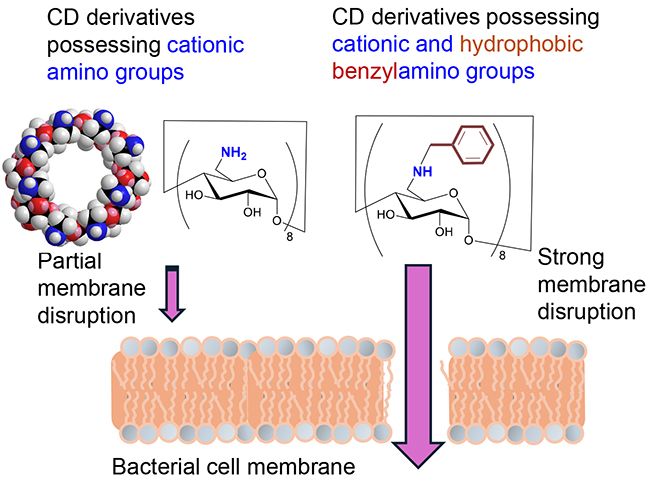
However, antimicrobial peptides are composed of various amino acids. If CDs are to mimic antimicrobial peptides in their original form, the structure of the CD derivative will be complex. Therefore, one of our objectives was to reproduce the antibacterial function of the peptides with the simplest possible CD structure. The other of our objectives was to examine the relationship between the structure and activity of functional groups and through this investigation discover ways to circumvent complicated synthesis with a view to practical application. Initially, only amino groups that interact with the membrane lipid phosphate were introduced into the CD molecule9. The application of the amino derivative to bacterial cultures resulted in the leakage of potassium ions from the inside of the bacterial cells, thereby confirming the damaging effect of the amino derivative on the bacterial membrane (Fig. 4). Nevertheless, the growth of bacteria was not inhibited, thereby demonstrating the necessity for a hydrophobic group capable of disrupting the interior of the lipid bilayer. Consequently, a benzylamino group possessing both cationic and hydrophobic functionality, was introduced into a glucose unit in the CD molecule by nucleophilic substitution9. The CD derivative with eight benzylamino groups was observed to be effective in damaging bacterial membranes. The minimum inhibitory concentration (MIC) required for the derivative to inhibit the growth of S. aureus was 5 μM, which is comparable to the value of the antimicrobial peptide gramicidin S (4 μM). In contrast to the typical antimicrobial peptides which typically possess numerous functional groups, the benzylamino CD derivatives demonstrated remarkable antibacterial efficacy despite their simplicity. This observation lends support to the concept of utilizing CDs to emulate the functions of antimicrobial peptides. Subsequently, we attempted to synthesize analogues with alternative amine nucleophiles with the objective of enhancing the antibacterial properties. However, despite our efforts, we were unable to find a suitable analogue that could be synthesized in good yields as all of the attempted analogues resulted in complex mixtures. It is plausible that this observed phenomenon can be attributed to the capacity of amine nucleophiles to initiate elimination reactions when they act as bases.
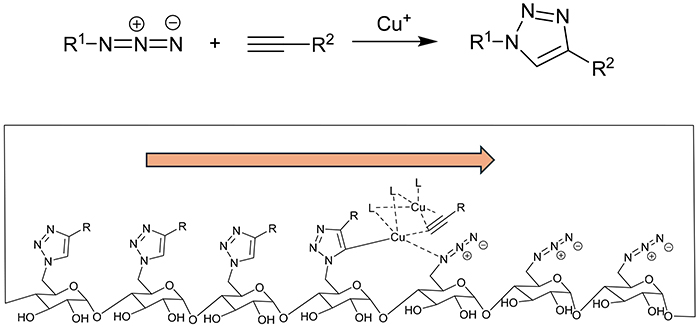
Accordingly, our research was directed towards the utilization of click chemistry, in lieu of nucleophilic substitution reactions, as a strategy for the introduction of membrane-disrupting functional groups. The reaction of an azide and an alkyne in the presence of a copper catalyst, which should yield the product "one click", was deemed optimal for the introduction of membrane-disordering groups into all the glucose units in the CD molecule. However, the standard heating method employed was unsuccessful in yielding the target product in a satisfactory quantity.
Hence, an investigation was conducted into the potential of microwave heating as an alternative method10. This approach involves molecular vibration, which allows for a more efficient internal heating of the reaction mixture. The results demonstrated that the use of microwave heating markedly enhanced the reaction efficiency, with the products reacting with all the glucose units of the CD in a relatively short period of time.
It is also noteworthy that even at the midpoint of the reaction, when the unreacted substance (the per-azide CD) was still present, only the target (the per-clicked CD) was generated and no intermediates (partially-clicked CDs) were observed. This "all-or-none" type reaction is thought to result from the formation of a complex among copper, alkyne, the azide group, and the triazole ring, which moves from one glucose unit to the next, proceeding as a chain reaction until all the azide groups on the molecule are consumed (Fig. 5). The highly efficient reaction also occurred in amylose composed of 1,000 glucose units with the poly-click reaction on all of the glucose units completed in period of 3 minutes11. Given the importance of the spacing and arrangement of the azide groups that cause the reaction, it is postulated that the microwave-assisted click reaction is not only effective in glucose-based amylose but also in oligosaccharides composed of other sugars. Consequently, the microwave-assisted click reaction is expected to be a valuable tool for the synthesis of various oligosaccharide derivatives.
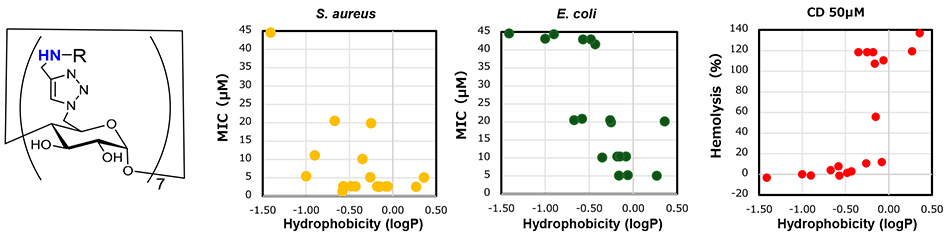
CD derivatives with diverse membrane-disordering groups were synthesized via microwave-assisted click reactions, with the objective of elucidating the correlation between structure and antibacterial properties. Initially, we synthesized CD derivatives in which alkylamino groups were introduced onto the primary hydroxy side of CD molecules12-15. The antibacterial activity was attributable to properties of the alkyl moiety. Alkyl groups with 6~7 carbon atoms conferred the strongest antibacterial activity. For example, the MIC for S. aureus was notably low, at 1~3 μM. Further investigation indicated that antibacterial activity was dependent on the hydrophobicity of the molecule, rather than the three-dimensional structure of the alkyl group, which had a chain-like, branched, or cyclic structural motif (Fig. 6). Furthermore, the hemolytic properties that result in the destruction of red blood cells, frequently observed in antimicrobial peptides, were also contingent upon hydrophobicity. However, the hydrophobicity that caused hemolysis was distinct from that which caused antibacterial activity. The discrepancy between the membrane disorder of bacterial cells and erythrocytes was postulated to be due to differences in the lipid and membrane structure of each cell membrane. It has been proposed that it is feasible for molecules to exhibit antibacterial activity selectively without damaging animal cells by controlling the hydrophobicity of the molecules. Derivatives of glucose and maltose exhibit minimal activity despite the presence of similar substituents. Consequently, a molecule with approximately seven or more substituents is highly desirable for its excellent antibacterial activity. It has been established that CD is an optimal material for antimicrobial substances11.
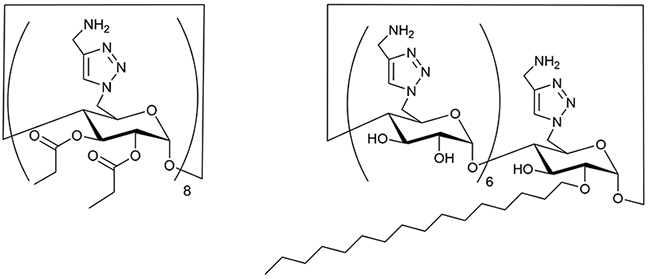
Subsequently, a derivative with an amphiphilic structure, in which the primary hydroxy side of CD is cationic and the secondary hydroxy side is hydrophobic, was synthesized (Fig. 7)16. This structure is analogous to that of the antimicrobial peptide gramicidin S, which features a cationic amino group situated on the upper side of the β sheet plane and a hydrophobic alkyl group on the lower side (Fig. 3). Among the CD derivatives in which amino groups and acyl groups were introduced, only those with propanoyl groups exhibited antibacterial properties. This phenomenon can also be explained by the hydrophobicity of the compound, a property that renders it antibacterial, as previously described. However, due to its relatively hydrophilic nature, the potential for hemolysis was reduced in comparison to the derivative that had the alkyl amino group as described above. Consequently, instead of acylating all the secondary hydroxy groups, a single long-chain alkyl group was attached17. This yielded a highly hydrophilic derivative with potent antibacterial activity and minimal hemolytic properties. By strategically configuring the functional group and positioning it on the CD, a novel derivative with selective antibacterial activity was successfully obtained. It should be noted here again that antimicrobial peptides are known to damage both animal cell membranes and bacterial cell membranes. The observed divergence between the CD derivative’s antibacterial effects and its toxicity resulting from damage to animal cell membranes could contribute to the development of a safer compound.
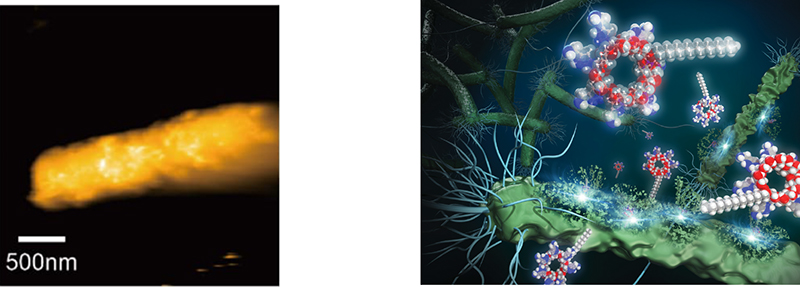
It was subsequently demonstrated that the antimicrobial mechanism of the CD derivatives is bacterial membrane disruption and is analogous to that of antimicrobial peptides. Gram-positive bacteria, such as S. aureus, possess a cytoplasmic membrane, whereas Gram-negative bacteria, including E. coli, have two membranes: a cytoplasmic membrane and a membrane outside of the cytoplasmic membrane (an outer membrane). The initial step involved evaluating the damage to the outer membrane of Gram-negative bacteria using the uptake of a fluorescent probe. This revealed that the damage was comparable to that observed with an antimicrobial peptide, polymyxin B11,15. Subsequently, a notable leakage of potassium ions from the bacterial cell was observed, which demonstrated disruption of the cytoplasmic membrane. Simultaneously, a rapid decline in the viability of bacteria was observed. These findings indicate that the CD derivative exerts its bactericidal action via bacterial cell membrane disruption13-17. As direct evidence of membrane disruption, we have succeeded for the first time in the world in capturing on video CD damage of the membrane surface of living E. coli cells using high-speed atomic force microscopy (AFM) (Fig.8)15.
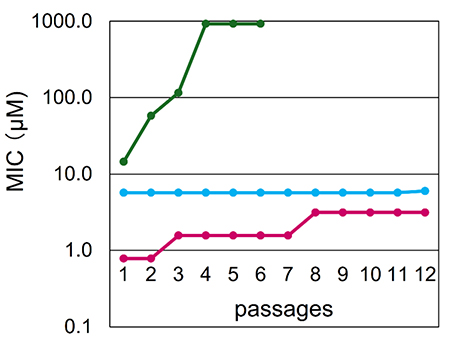
The antibacterial CD derivatives have been demonstrated to damage bacterial membranes, and thus were expected to exhibit antibacterial activity not only against susceptible bacteria but also against antibiotic-resistant bacteria. Experimentally, we demonstrated that the derivatives were effective against drug-resistant strains isolated clinically. For example, the CD derivative MIC for MRSA, which has gained notoriety for causing nosocomial infections, was 2 μM and nearly identical to that of the vancomycin MIC (1 μM), a medication utilized for the treatment of MRSA-related infections. Moreover, the MICs for E. coli strains that produce substrate-specific extended β-lactamases and those that produce carbapenemases, which are classified as very serious pathogens by the World Health Organization (WHO), the U.S. Centers for Disease Control and Prevention (CDC), and the Japan Agency for Medical Research and Development (AMED) Public and Private Partnerships for Infectious Diseases Research and Development, are 4 μM and 6 μM, respectively. The MICs were found to be almost identical to that of polymyxin B, which is a multidrug-resistant drug used for the treatment of Gram-negative bacterial infections and is designated by the WHO as an essential medicine18. With regard to resistance, resistance to the antibiotic fosfomycin, which inhibits cell wall synthesis, was demonstrated to have increased 64-fold in comparison to the original MIC, while resistance to norfloxacin, which inhibits DNA synthesis, was shown to have increased 30-fold. It is noteworthy that the MIC of the CD derivative with amino and propanoyl groups did not undergo any significant alteration (Fig. 9)16. It can be reasonably deduced that the development of resistance to CD derivatives that cause bacterial membrane damage is improbable, and that the risk of drug resistance can be expected to be low. It seems reasonable to suggest that the practical application of CD derivatives exhibiting antibacterial properties against antibiotic-resistant bacteria will prove invaluable in the fight against pathogenic bacteria. The new agents will have the potential to save numerous lives over an extended period of time, thus avoiding the problem of immediate ineffectiveness due to the emergence of antibiotic-resistant bacteria.
As described above, our research was inspired by antimicrobial peptides and we have developed CD derivatives with potent antibacterial properties by utilizing the unique properties of CD molecules. Our research has revealed a distinctive and versatile method for chemical modification of CD molecules. The systematically synthesized derivatives demonstrate that there is a relationship between antibacterial properties and structure, and based on their performance, CD derivatives can be expected to be put to practical use. These studies show that the oligosaccharide CD can be chemically modified to express novel functions and is of great interest from an academic and practical standpoint.