
Hiroyuki Kono
Professor, National Institute of Technology, Tomakomai College, Japan
Professor, National Institute of Technology, Tomakomai College, Japan
He received his MD from Hokkaido University in 1995, joined the Hokkaido Research Organization, Industrial Research Institute , and then moved to Bruker Japan K.K. During that time, he received his PhD from Hokkaido University in 2003. He was appointed Associate Professor at the National Institute of Technology in 2007 and was promoted to Professor in 2019. He engages in functionalization of structural polysaccharides (mainly cellulose and chitin and chitosan) and elucidation of their structure-function relationship. Awards: Hayashi Jisuke Award from the Cellulose Society of Japan (2017), Technology Development Award from the Japanese Society of Applied Glycoscience (2020), etc.
Hydrogels can generally be synthesized from chitosan by physical cross-linking or chemical cross-linking, but the chemical cross-linking method is simpler to use and yields a structurally more stable product. Chemical cross-linking agents such as glutaraldehyde and epichlorohydrin are widely used for the preparation of polysaccharide-based hydrogels, but there are concerns about environmental and biological toxicity. Although natural cross-linking agents such as genipin1, which is a chemical compound found in Genipa americana fruit extract, are available, their industrial use is limited by cost. Recently, it was reported that polyaldehydes, obtained by oxidizing polysaccharides2‒5 or oligosaccharides6,7, can be used as a cross-linking agent, and their biological safety and other properties are being confirmed8,9. In this article, we focus on oxidized sucrose (OS), which is obtained by oxidative cleavage of inexpensive sucrose, and its molecular structure and properties as a cross-linking agent for chitosan and its derivatives.
Hydrogels form three-dimensional networks of hydrophilic polymers in which individual polymer chains are chemically or physically cross-linked, allowing them to absorb water or saline solutions and retain large volumes of these solutions without deforming their structure. Because of their high water content and flexibility similar to natural tissues, hydrogels are widely used in biomedical and pharmaceutical processes such as drug and gene delivery, tissue engineering, healing, and cosmetic manufacturing10‒13. Among the various matrices employed in hydrogel preparation, chitosan and its derivatives have attracted worldwide attention as biomaterials due to their excellent properties such as non-toxicity, biocompatibility, biodegradability, bioactivity, antibacterial properties, and adhesive properties. Chitosan hydrogels, which can be injected into the body, have recently attracted considerable attention, and are becoming effective tissue engineering tools that offer a simple and minimally invasive alternative to surgical implantation14,15. However, there are various challenges to overcome, such as the toxicity of cross-linking agents and reagents used in the process of hydrogel preparation and cleaning.
Periodate oxidation of polysaccharides and oligosaccharides under acidic conditions results in partial oxidative cleavage of carbon-carbon bonds to form polymeric aldehydes. As an example, the oxidation of cellulose by sodium periodate cleaves the C2‒C3 bond of an anhydroglucose residue to produce 2,3‒dialdehyde cellulose2. Similarly, polyaldehydes are obtained from sodium alginate, dextran, hyaluronic acid, and pectin by the similar oxidation process3‒5. However, polysaccharide polyaldehydes have the problem of insufficient cross-linking efficiency; although they have a large number of aldehyde groups in their structure that serve as cross-linking points, very few aldehyde groups form cross-links because of their high molecular weight and the bulkiness of their backbone structure. In contrast, OS, obtained by oxidizing sucrose, has high water solubility and low molecular weight, which allow it to be treated in a similar manner to existing chemical cross-linking agents such as glutaraldehyde. For example, OS has been reported to efficiently cross-link proteins, such as collagen, gelatin, and zein from corn protein, to enhance the physical and chemical properties of these biomaterials8,16,17. In addition, OS is a non-volatile polar molecule that does not exhibit environmental toxicity. Furthermore, the biocompatibility of OS has been also demonstrated by in vitro cell culture studies of protein scaffolds cross-linked by OS8.
OS is obtained simply by adding sodium periodate to an aqueous sucrose solution (Fig. 1). Formic acid and sodium iodate are produced as reaction byproducts, which can be removed by ion exchange resin treatment and precipitation by barium salt addition to the reaction solution, respectively. Finally, the reaction solution is lyophilized to obtain a white powdered OS.
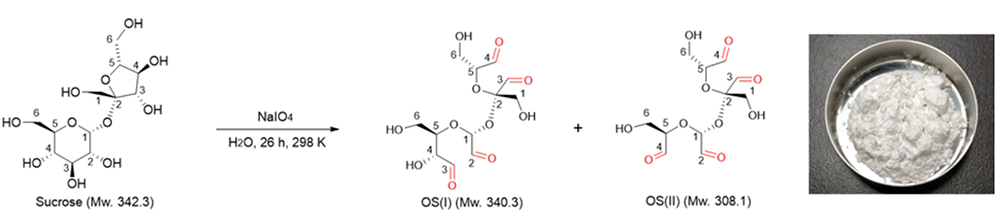
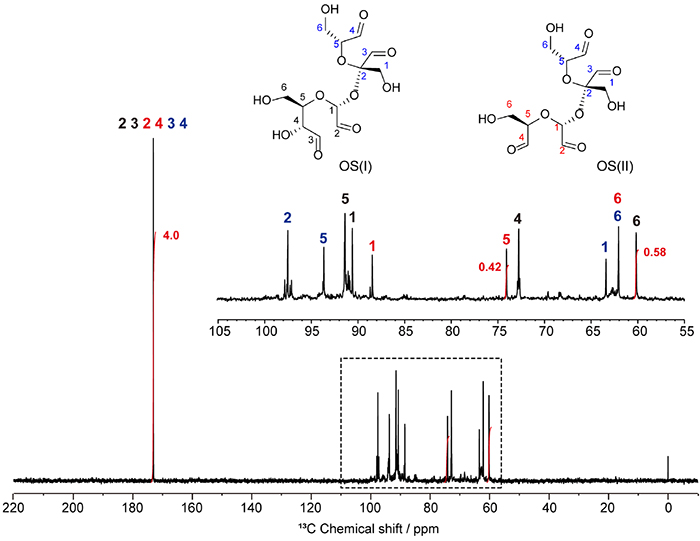
The products of sucrose oxidative cleavage are considered to be a complex isomeric mixture of acetals in which multiple carbon-carbon bonds of fructose and glucose residues in sucrose are oxidatively cleaved. Recently, the authors attempted to precisely determine the molecular structure of OS and found that it is a mixture of two tetra-aldehyde derivatives, OS(I) and OS(II) (Fig. 1) 18. Both of them showed oxidative cleavage of the C2‒C3 bond of the fructose residue, and the products of oxidative cleavage of the glucose residue were different: OS(II) was the product of complete oxidative cleavage at the C2‒C3‒C4 bonds, whereas OS(I) was the product of partial oxidative cleavage at the C2‒C3 bond. Unfortunately, OS(I) and OS(II) are similar in structure and molecular polarity, and have not yet been isolated and purified.
The proportion of OS(I) to OS(II) in the mixture can be determined by quantitative 13C NMR. As shown in Figure 2, the integral value for the C5 resonance of the fully oxidatively cleaved glucose residue of OS(II) at 74 ppm and that of the C6 resonance of the partially oxidatively cleaved glucose residue of OS(I) at 60 ppm, which do not overlap with other resonances, were 0.42 and 0.58, indicating that the compositions of OS(I) and OS(II) were 52 mol% and 48 mol%, respectively. In addition, the integral value of 3.96 obtained for the aldehyde carbon atoms confirmed that both OS(I) and OS(II) possess four aldehyde groups in their structures.
As a polycation with a high density of positive ammonium groups, chitosan dissolves in acidic solutions but is not soluble at neutral or physiological pH. On the other hand, carboxymethyl chitosan (CMC), in which some of the amino and hydroxyl groups of chitosan are carboxymethylated, is readily soluble in the neutral pH range and is widely used in wound healing, bioimaging, tissue engineering, and drug- and gene-delivery systems. CMC as well as chitosan are degraded by enzymes in the human body, such as lysozyme and chitosanase, to produce chitooligo- and monosaccharides, which are subsequently absorbed in the human body.
When OS is added to a CMC aqueous solution, a cross-linking reaction proceeds without catalyst, resulting in gelation; the synthesis of hydrogels (Table 1, CMCG1‒3) obtained by adding OS at 0.5, 1.0, and 2.0 moles of aldehyde groups relative to unsubstituted amino groups in CMC are shown below as an example.
| Hydrogel | CMC/mg (NH2/mmol) | OS/mg (CHO/mmol) | Cross-linking density a | CMCG 1 | 400 (0.14) | 6.10 (0,07) | 0.08 | CMCG 2 | 400 (0.14) | 12.2 (0.14) | 0.11 | CMCG 3 | 400 (0.14) | 24.5 (0.28) | 0.14 |
|---|
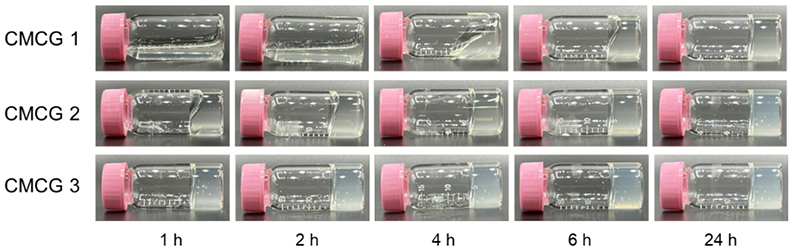
As shown in Fig. 3, CMC solution changes from a sol to a gel immediately after the reaction. The cross-linking rate depended on the OS concentration, and complete gelation occurred within 3 h under CMCG 3 synthesis conditions. For CMCG 1 and 2, the rheological behavior stabilized within 24 h, indicating that the cross-linking reaction between CMC and OS is completed within 24 h. The resulting gels can be washed with water and then lyophilized to obtain white powdered CMCG samples.
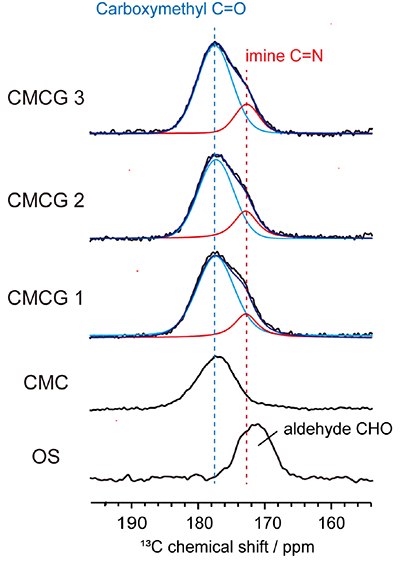
In the carbonyl carbon region of the quantitative solid-state 13C NMR spectra of CMCG samples, a new resonance assigned to imine carbon can be detected in addition to resonance for carboxymethyl group of CMC (Fig. 4). Since the carboxymethyl group does not contribute to the cross-linking process with OS, the number of imine bonds formed per anhydroglucosamine residue, i.e., the cross-linking density of CMCG, can be determined by applying Lorentzian lineshape fitting analysis to the carbonyl carbon region of the spectra (Table 1).
Cross-linking by OS does not proceed for acidic or neutral polysaccharides such as carboxymethyl cellulose, but only for polysaccharides containing amino sugars such as glucosamine and galactosamine. In other words, it can be concluded that the cross-link formed in CMC by OS is an imine bond between the amino group of CMC and the aldehyde of OS (Fig. 5). The immersion of the obtained hydrogel in sodium borohydride solution can also reduce the imine to a stable secondary amine and the unreacted aldehyde group of OS to a hydroxyl group, respectively.
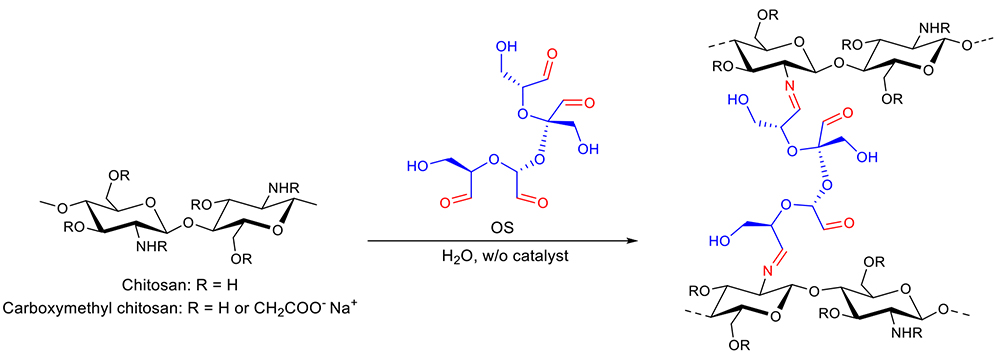
When CMCGs contact water molecules, the sodium carboxymethyl groups ionize to form a polyanion. The electrostatic repulsion between the CMC chains causes the gel to swell, and at the same time, the free sodium ions generate osmotic pressure, which leads to water absorption. Once water is absorbed, it is not released even under pressure due to intermolecular cross-linking by the OS. Figure 6 shows the saturated absorbency of CMCG 1‒3 in pure water and in phosphate-buffered saline (PBS, pH 7.4) solution. Comparing the water absorption of CMCGs in water and PBS, the water absorption decreases because the salt in PBS suppresses the osmotic pressure in the CMCG gel. In addition, the increase in cross-link density suppresses the electrostatic repulsion between CMC molecules, resulting in lower water absorption.
The SEM images of cross-sections of the lyophilized CMCG 1‒3 after saturation absorption in PBS showed that these hydrogels had a cellular structure consisting of macropores. The sizes of these macropores depended on the extent of cross-linking (CR); a higher CR led to a decreased macropore size19, which is consistent with the result that an increase in the CR resulted in a decrease in the water absorbency (Fig. 6).
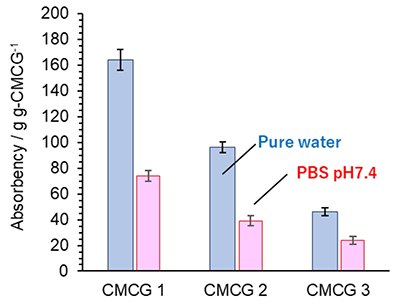
The dynamic viscoelasticity of the CMCG samples swollen in PBS was evaluated from a graph of the elastic modulus against the angular frequency sweeps (Fig. 7); both the storage modulus (G’) and loss modulus (G”) of CMCGs exhibited constant values regardless of the CMCG. In addition, the value of G' was 10 times higher than that of G” for all samples, and there was no inter-section between the plots for G’ and G”. These observations indicate that the CMCG samples were typical chemical gels with the characteristics of a permanent gel network. Furthermore, the rheological measurements show that an increase in the CR resulted in higher G’ and G” values, ultimately leading to an increase in ƞ*. Therefore, it was apparent that the inclusion of a large amount of OS caused a significant increase in the G’ and G” moduli and in ƞ*.
When synthesizing hydrogels from chitosan using OS, the cross-linking reaction can be performed in an acidic aqueous solution. In an acidic aqueous solution, the amino groups of chitosan are transformed to ammonium cations. The protonation of the amino group enhances the nucleophilicity of the nitrogen atom, which increases the reactivity of OS toward the aldehyde carbon. As a result, the reaction rate of cross-linking between chitosan chains via OS molecule is extremely fast. The obtained chitosan hydrogels have no ionic functional groups such as carboxymethyl groups, and, therefore, the water-absorbency of chitosan hydrogel is generally low compared to that of CMCG.
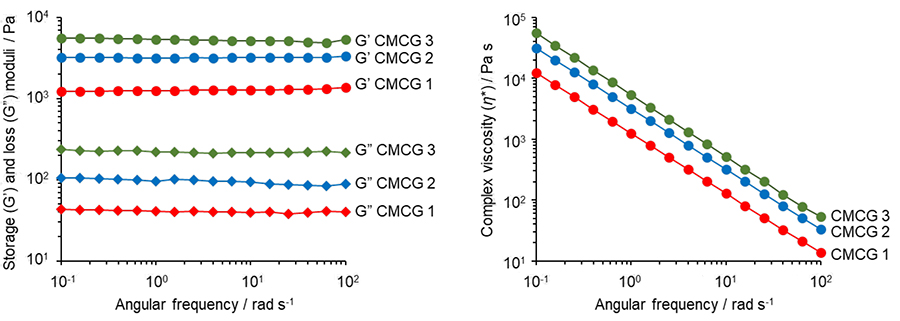
Figure 8 (a) shows the relationship between the gel strength (storage modulus) and reaction time that occurs when a certain amount of OS is added to a chitosan solution at concentrations of 2, 3, and 4 w/v%. When the initial concentration of chitosan is high (4 w/v%), the cross-linking reaction proceeds rapidly immediately after the start of the reaction, resulting in the formation of an extremely high-strength hydrogel. Focusing on the features of rapid and high-strength gelation performance of chitosan hydrogel, we are investigating its application to bioadhesives. As an example, we have confirmed that a drop of aqueous chitosan solution and aqueous OS solution on the surface of porcine skin develops strong adhesion to a second porcine skin surface within 5 min (Fig. 8 (b)). Therefore, if the non-toxicity, non-antigenicity, and other biological safety properties of OS are confirmed, it may be possible to develop various medical applications such as injectable hydrogels.
In this paper, we introduce a method for controlling the structure and properties of biological hydrogel materials utilizing the functionality of chitosan, which is one of the themes of our work on the development of carbohydrate-based functional materials. OS obtained from inexpensive sugars has the potential to solve the problem of cross-linking agent toxicity, which has been a concern in the past. Therefore, by applying OS to polysaccharides containing amino sugars such as chitosan, hyaluronic acid, heparin, and chondroitin sulfate, the resulting gel materials may be used for pharmaceutical applications. Owing to its excellent formability into fibers and beads and its metal adsorption capacity, chitosan is also being applied to the materials field. In addition to the technologies described in this paper, there are also technologies and expertise in preparation of nano- to millimeter-scale spherical gel particles, and functionalization of polysaccharides such as surface modification, chemical modification, and composite formation with other functional materials, and structural characterization. By integrating these technologies, we hope to maximize the inherent functionality of chitosan.