Maiko Uehara
Department of Chemistry and Life Science, School of Advanced Engineering, Kogakuin University
Kogakuin University, Tokyo, Japan, B.S. (Engineering), 2017
Kogakuin University, Tokyo, Japan, M.S. (Engineering), 2019
Kogakuin University, Tokyo, Japan, Ph.D. (Engineering), 2022

Fumitaka Oyama
School of Advanced Engineering, Kogakuin University
Ibaraki University, Ibaraki, Japan, B.S. (Agricultural Chemistry), 1980.
Tohoku University, Miyagi, Japan, M.S. (Agricultural Chemistry), 1982.
Tohoku University, Miyagi, Japan, Ph.D. (Agricultural Chemistry), 1985.
Postdoctoral fellow, Mitsubishi Kasei Institute of Life Sciences, Tokyo, Japan, 1985-1987.
Instructor, Institute of Comprehensive Medical Science,Fujita Health University, Aichi, Japan, 1987-1992.
Assistant Professor, Graduate School of Medicine, University of Tokyo, Tokyo, Japan, 1992-2002.
Research Scientist, RIKEN Brain Science Institute, Saitama, Japan, 2002-2009.
Professor, Department of Applied Chemistry, Faculty of Engineering, Kogakuin University, Tokyo, Japan, 2009-2015..
Professor, Department of Chemistry and Life Science, School of Advanced Engineering, Kogakuin University, Tokyo, Japan, 2015-present
Dean, School of Advanced Engineering, Kogakuin University,Tokyo, Japan, 2021-present.
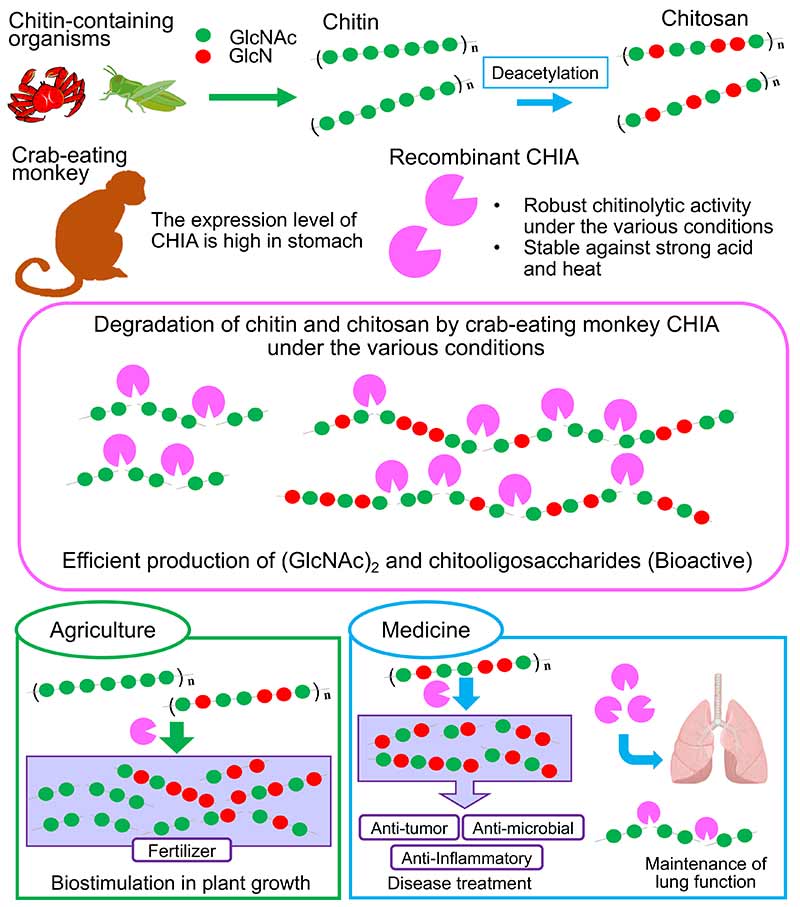
Chitooligosaccharides produced by degrading chitin or chitosan possess attractive biological activities such as anti-bacterial, anti-tumor and anti-inflammatory effects. The use of enzymes in the production of chitooligosaccharides may increase their value in terms of safety and simplicity of process control. We have shown that the monkey expresses a high level of CHIA mRNA in the stomach. Crab-eating monkey CHIA had robust chitinolytic activity under a broad range of pH conditions with high thermal stability. In addition, crab-eating monkey CHIA efficiently degrades chitin and chitosan to produce chitooligosaccharides under acidic and high-temperature conditions without inactivation. We propose crab-eating monkey CHIA for application in agricultural and biomedical purposes.
Chitin is a β-1,4-linked polymer of N-acetyl-D-glucosamine (GlcNAc). It is an integral component of the exoskeletons of crustaceans and insects, the microfilarial sheaths of parasitic nematodes and fungal cell walls1,2. Thus, this polysaccharide is the second most abundant polysaccharide in nature. Chitosan is a liner amino polysaccharide composed of D-glucosamine (GlcN) and GlcNAc units. It is deacetylated derivative of chitin.
Chitooligosaccharides are degradation products from chitin or chitosan. Chitooligosaccharides have remarkable bioactivities such as anti-microbial, anti-tumor effects, anti-inflammatory and drug delivery3-6. In addition, chitooligosaccharides also function as biostimulators in plant growth and are used as biopesticides and biofertilizers in agriculture7,8.
Chitinases are enzymes that hydrolyze the chitin polymer. Although mammals do not produce chitin, mice and humans synthesize two active chitinases, chitotriosidase (CHIT1) and acidic chitinase (CHIA)2,9,10. Previous studies have shown associations between CHIA expression and specific pathophysiological conditions. CHIA is associated with bronchial asthma in humans11-15. Recent studies using Chia-deficient mice have shown that Chia is a constitutively produced enzyme necessary for chitin degradation in the airways to maintain lung function [10, 16]. In addition, Chia has been shown to possess functions as a protease-resistant chitinase under gastrointestinal conditions17-20.
The crab-eating monkey (Macaca fascicularis) is one of the nonhuman primates and the most crucial nonhuman primate animal models for biomedical research21-23. By the term "crab-eating monkey," these primates feed on crabs and other chitin-containing organisms such as crustaceans and insects. Thus, we expected that crab-eating monkey has strong chitinase because they eat crabs. In 2016, we started the present research on crab-eating monkey chitinase.
We introduced gene expression analysis, enzymatic properties, and potential application for chitooligosaccharide production by crab-eating monkey acidic chitinase (CHIA).
We performed gene expression analysis of chitinases in crab-eating monkey tissues by quantitative real-time PCR (qPCR). The total RNA from various crab-eating monkey tissues was analyzed. The highest levels of CHIA mRNA were detected in the stomach; CHIA was a major transcript in the crab-eating monkey stomach (Figure 1).
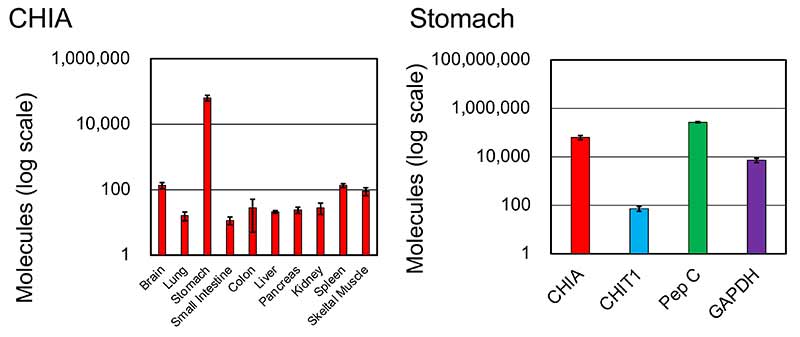
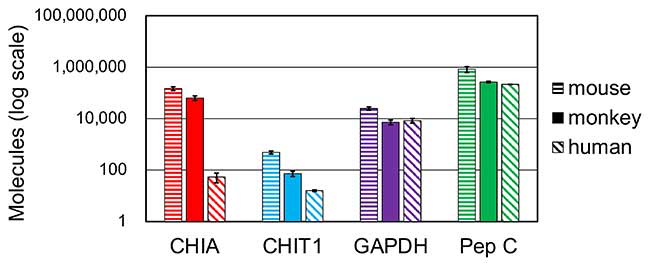
Next, we carried out cross-species gene expression analysis to compare mouse, monkey, and human total stomach RNA. The highest expression levels of CHIA mRNA were observed in mouse, followed by monkey and human. The expression of CHIA in monkey and mouse was remarkably higher than in human. Therefore, CHIA was expressed predominantly in crab-eating monkey stomach (Figure 2).
Next, we analyzed the enzymatic activity of the chitinases in the stomach or lung tissue extracts of mouse, crab-eating and human stomach. We detected strong chitinolytic activity from crab-eating monkey stomach extracts that expressed CHIA mRNA highly (Figure 3). Thus, the difference in mRNA reflected the chitinolytic activity of tissue extracts.
These results suggested crab-eating monkey CHIA has strong chitinolytic activity24.
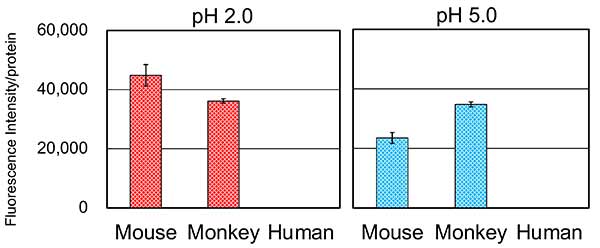
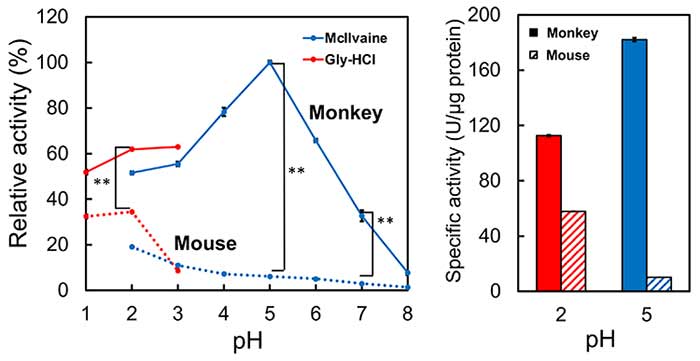
We expressed crab-eating monkey CHIA as a recombinant protein in E. coli. We examined the pH dependence of crab-eating monkey CHIA. Crab-eating Monkey CHIA achieved the highest activity at pH 5.0 and remains active at pH 1.0–7.0 (Figure 4, left). Activities of monkey CHIA were two times higher at pH 2.0, 16 times higher at pH 5.0, and 10 times higher at pH 7.0 than those of mouse Chia (Figure 4, left). There was a threefold difference in their peak activities crab-eating monkey CHIA at pH 5.0, mouse Chia at pH 2.0 (Figure 4, right).
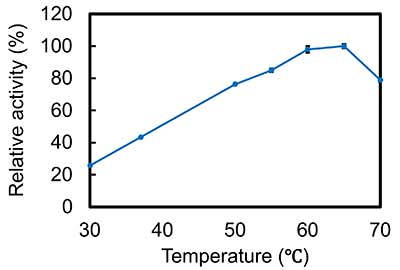
We determined the effect of temperature on enzyme activity at pH 5.0 (optimal pH of crab-eating monkey CHIA). The optimal temperature was 65℃. The crab-eating monkey CHIA maintained its high activity even at 70℃ (Figure 5).
Moreover, crab-eating monkey CHIA had acid- and thermo- stabilities. Thus, crab-eating monkey CHIA had robust chitinolytic activity under a broad pH and temperature range25.
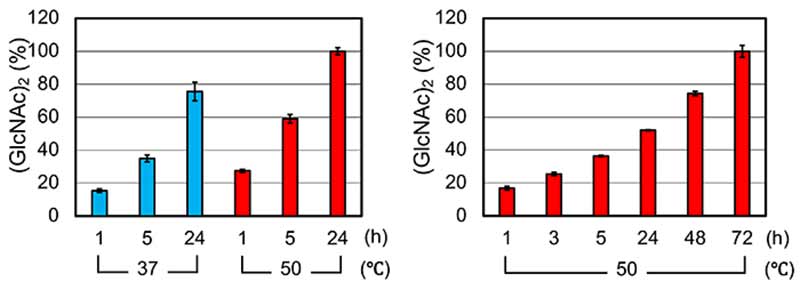
We compared chitin degradation under the physiological (37℃) and high-temperature conditions (50℃) because crab-eating monkey CHIA was stable until 70°C. The chitin degradation was higher efficiency at 50℃ than 37℃ (Figure 6, left). In addition, the levels of chitooligosaccharides grew during the whole incubation, indicating no inactivation of CHIA for long time incubation at 50℃ (Figure 6, right).
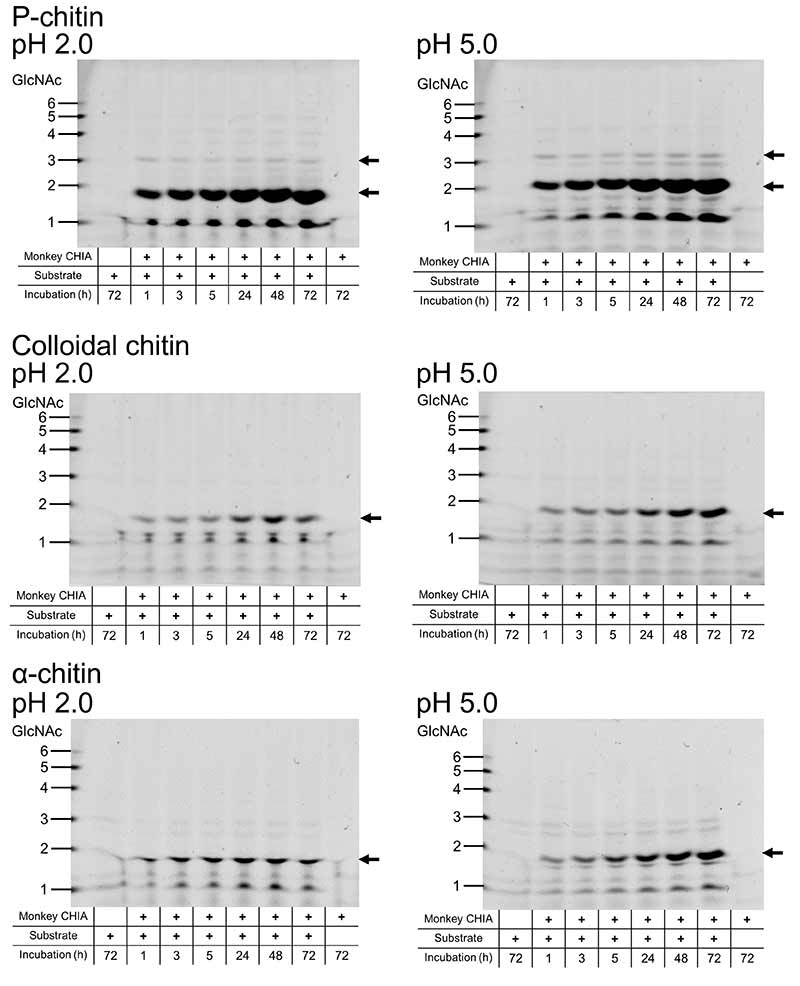
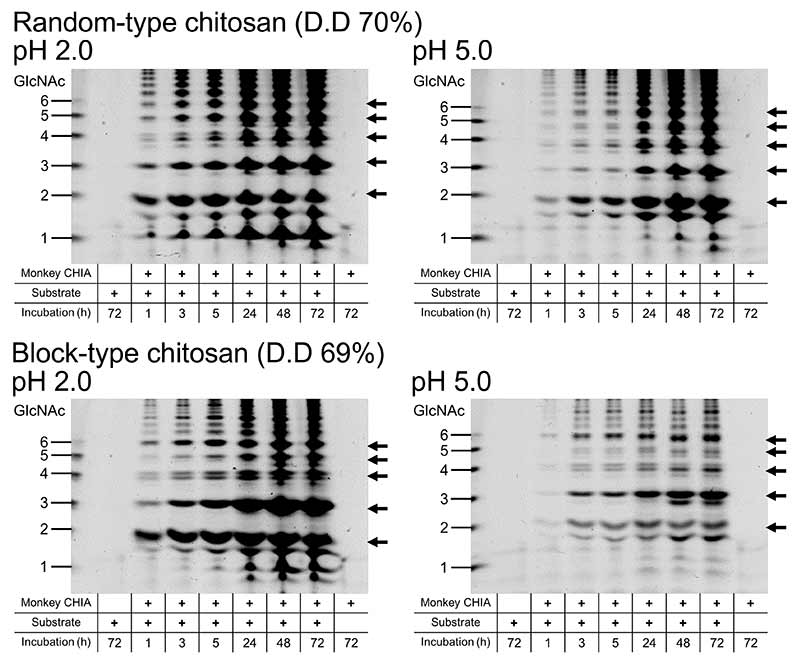
Next, we incubated chitin substrates (α-chitin, colloidal chitin and P-chitin) and chitosan substrates (block-type and random-type chitosan) with crab-eating monkey CHIA. We analyzed the degradation products by the fluorophore-assisted carbohydrate electrophoresis (FACE) method18,19,24,26,27. Crab-eating monkey CHIA produced (GlcNAc)2 mainly from chitin substrates. (GlcNAc)2 levels produced by crab-eating monkey CHIA were increasing with incubation time. (GlcNAc)2 were produced at the highest level from P-chitin (Figure 7).
The various chitooligosaccharides were produced from chitosan substrates. The degradation products increased with incubation time and changed the pattern of produced chitooligosaccharides. By modifying the substrate and pH, it is possible to control the chitin and chitosan degradation pattern. In addition, the degradation products of random-type chitosan were produced in large amounts compared with block-type chitosan (Figure 8)28.
Chitooligosaccharides are produced either by chemical degradation or enzymatic hydrolysis of chitin and chitosan. Enzymatic preparation methods using mammalian sources enhance the products' value due to their safety and control of the process. We defined monkey CHIA had strong activity than mouse Chia significantly25. The chitinolytic activity of monkey CHIA is high under various conditions with thermo- and acid-stability. We showed degradation of chitin and chitosan substrates to produce oligosaccharides using the most robust mammalian chitinase, crab-eating monkey CHIA. Crab-eating monkey CHIA can be used to produce low-to-moderate length chitooligosaccharides, promoting plant growth for agricultural applications7,8 (Figure 9).
The studies in Chia-deficient mice have shown that chitin polymers accumulate in the airways, causing pulmonary fibrosis that can be ameliorated by administering active enzymes10-16. In humans, it was reported CHIA was related to bronchial asthma11-15. Increasing chitinase activity in humans could be a preventive and/or therapeutic goal for lung disease. Due to its high homology with human CHIA, crab-eating monkey CHIA might potentially be adjusted for future purposes (Figure 9). Crab-eating monkey CHIA could therefore become a robust tool in chitooligosaccharide production for agricultural and biomedical purposes (Figure 9) 28 .