
Jun-ichi Kadokawa
Professor, Graduate School of Science and Engineering, Kagoshima University, Japan
He studied applied chemistry and materials chemistry at Tohoku University, where he received his Ph.D. in 1992. He then joined Yamagata University as a Research Associate. From 1996 to 1997, he worked as a visiting scientist at the Max Planck Institute for Polymer Research in Germany. In 1999, he became an Associate Professor at Yamagata University and moved to Tohoku University in 2002. He was appointed as a Professor of Kagoshima University in 2004. His research interests focus on synthesis, functionalization, and controlled assembly of polysaccharides. He received the Award for Encouragement of Research in Polymer Science (1997), the Cellulose Society of Japan Award (2010), the Royalty Award from the Institute of Materials, Malaysia (2016), and IAAM Medal (2016). He has published more than 240 papers in academic journals.
Chitin, like cellulose, is one of the most abundant organic materials on earth1-3. However, most of it is unutilized as a practical material due to its crystalline structure, which is highly fibrous, and its extended molecular chain packing, which is stiffened by numerous intra- and intermolecular hydrogen bonds. Particularly acetamido groups in repeating N-acetyl-ᴅ-glucosamine units form quite strong intermolecular hydrogen bonds, posing more serious solubility and processability problems than does the hydrogen bond structure of cellulose. Recently, ionic liquids have been identified as media suitable for the fabrication of chitin-based soft materials4-8. This article summarizes the contributions made to the development of soft-materials from chitin, such as flexible, thermoplasticized materials, and so on, through dissolution and gelation in ionic liquids.
Ionic liquids are molten salts with melting points lower than those of water. Their use as powerful solvents for polysaccharides9-11 has been recognized since Rogers et al. found that cellulose would dissolve in the ionic liquid, 1-butyl-3-methylimidazolium chloride (BMIMCl)12. The large size (mainly cations) and conformational flexibility of the ions, leading to small lattice enthalpies and large entropy changes, favor the liquid state13. Some ionic liquids, such as 1-ethyl- and 1-butyl-3-methylimidazolium acetates (EMIMOAc and BMIMOAc) have been reported to dissolve chitin (Fig. 1)14,15. The author also found that chitin will dissolve in another ionic liquid, 1-allyl-3-methylimidazolium bromide (AMIMBr) (Fig. 1)16.
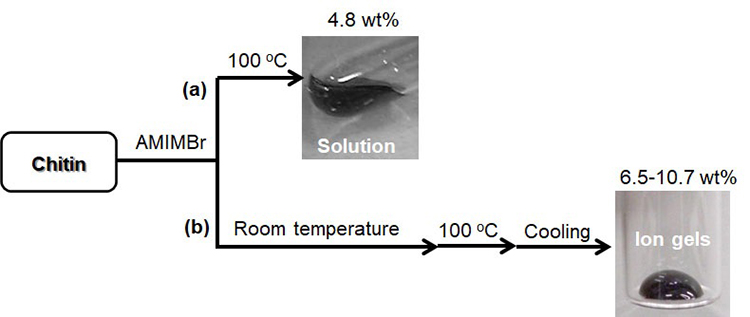
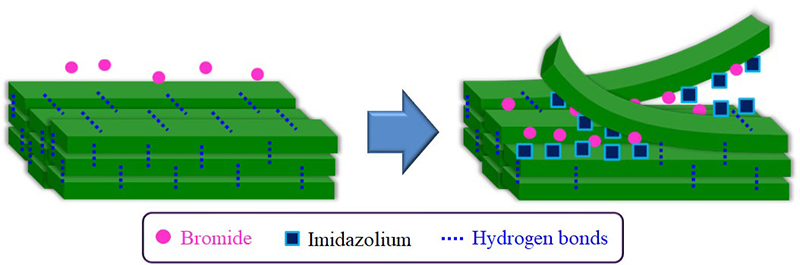
The dissolution of chitin in BMIMOAc was first reported in 200814. The chitin/BMIMOAc solutions were changed to the gel state by cooling to room temperature and were further converted into chitin sponge and film materials by regeneration using a water or methanol coagulant. Certain concentrations of EMIMOAc and the other imidazolium ionic liquids with carboxylate anions, which are similar in structure to BMIMOAc, were also reported to dissolve chitin15,17,18. The author has found that another ionic liquid, AMIMBr, dissolves chitin at concentrations of up to 4.8 wt% by heating at 100 °C (Fig. 2[a])16. Molecular dynamics simulations were performed to evaluate the dissolution behavior of chitin crystals in AMIMBr, which suggested that Br- contributed to the cleavage of intermolecular hydrogen bonds, whereas AMIM+ prevented the return to the crystalline phase after peeling (Fig. 3)19. When larger amounts of chitin (6.5-10.7 wt%) were successively immersed in AMIMBr at room temperature, heated at 100 °C, and cooled to room temperature, highly viscous materials, i.e., ion gels were formed (Fig. 2[b])16.
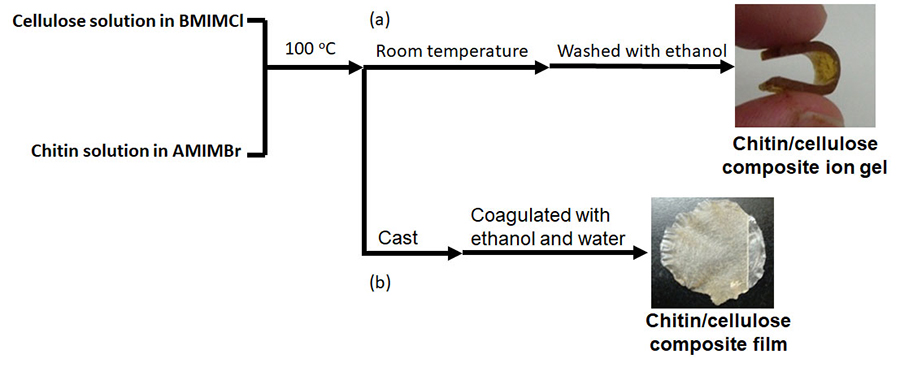
A chitin/cellulose composite ion gel was fabricated by using a homogeneous solution containing two ionic liquids, AMIMBr and BMIMCl; the solution was prepared by mixing the individual ionic liquids at 100 °C, leaving the solution to cool at room temperature to eliminate excess ionic liquids, and then washing with ethanol (Fig. 4[a])20.
Chitin-based soft materials, such as films and hydrogels, have been fabricated through dissolution in ionic liquids. As mentioned above, chitin sponge and film have been regenerated from chitin ion gels with BMIMOAc using water and methanol as coagulants, respectively14. EMIMOAc has also been used to produce hydrogels, films, and membranes from chitin21-24.
Chitin-containing composite soft materials have been fabricated using ionic liquids. For example, chitin/cellulose composite films and hydrogels have been made by using imidazolium carboxylates25-27. Chitin/cellulose composite hydrogels, which were obtained using EMIMOAc, were employed as novel electrolytes for electrochemical capacitors28. The abovementioned chitin/cellulose composite ion gel fabricated by using AMIMBr/BMIMCl was also investigated as an electrolyte for an electric double-layer capacitor after conversion into the corresponding acidic gel by treatment with a 2.0 mol/L H2SO4 aqueous solution29-31. The chitin/cellulose homogeneous solution in AMIMBr/BMIMCl was cast on a plate and subsequently coagulated with ethanol and water to produce a chitin/cellulose composite film (Fig. 4[b])20,32.
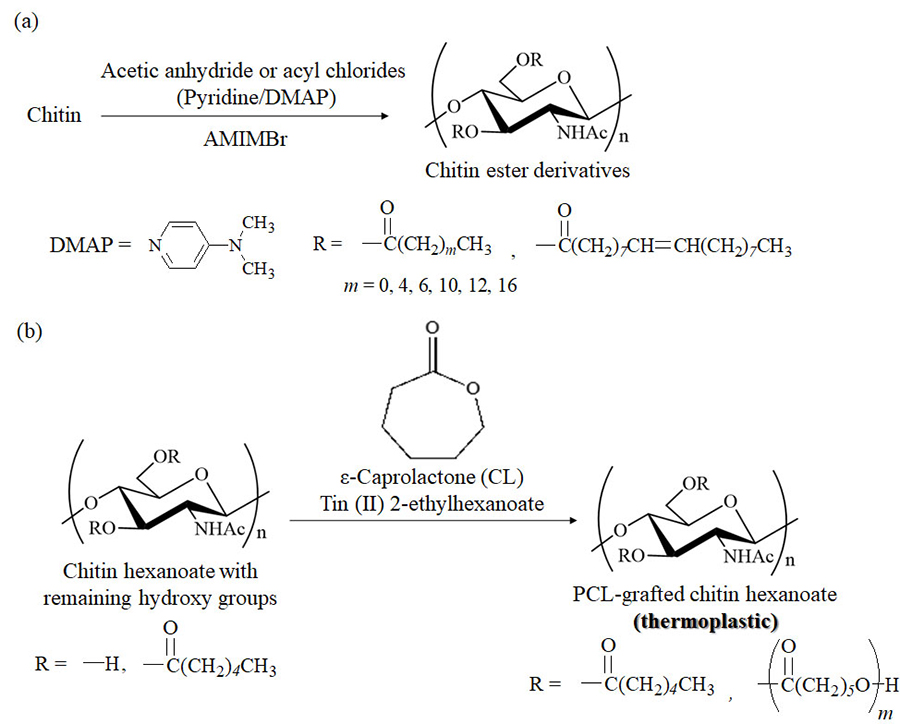
Hydroxy group modifications, such as acylation, are well-known functional materialization approaches used to convert cellulose into practical cellulose ester derivatives33-35. Chitin ester derivatives, however, are applied as practical materials because efficient acylation methods for chitin have not been sufficiently developed. The author has reported that AMIMBr is a good reaction medium for chitin acylation using acetic anhydride and various acyl chlorides to produce chitin ester derivatives with high degrees of substitution (DSs) under suitable conditions (in the presence of pyridine/N,N-dimethyl-4-aminopyridine [DMAP], if necessary) (Fig. 5[a])36-38. Heating a mixture of hexanoyl chloride with AMIMBr at 100 °C causes halogen exchange between two substrates to produce hexanoyl bromide in situ (Fig. 6). This result suggests that acyl bromides, because they are more reactive than acyl chlorides, efficiently react with hydroxy groups in chitin to form high-DS products. By contrast, imidazolium carboxylates, such as EMIMOAc and BMIMOAc, are not considered as suitable media for chitin acylation, because the higher basicity of carboxylates compared to that of bromide in AMIMBr leads to potential reactivity with acylation reagents. The synthesized single chitin esters containing a kind of substituent, prepared in AMIMBr, however, did not exhibit good material processability and handling properties, such as film formability. Mixed chitin esters with two different substituents, that is, stearoyl and some long and bulky acyl groups, were then synthesized in AMIMBr using the same acylation procedure to provide derivatives with specific processability39. All products showed film formability by casting from solutions in chloroform or chloroform/trifluoroacetic acid solvents, but thermoplasticity was not observed.
To prepare thermoplastic chitin derivatives, thermoplastic polymer grafting was carried out by surface-initiated ring-opening graft polymerization of ε-caprolactone (CL) from the remaining hydroxy groups in chitin hexanoates, which were prepared by the acylation of chitin with hexanoyl chloride in AMIMBr according to the abovementioned method (Fig. 5[b])40. The produced poly(ε-caprolactone) (PCL)-grafted chitin hexanoates mostly contained uncrystallized chitin chains, and furthermore, longer PCL graft chains formed crystalline structures. Accordingly, the PCL graft chains in the products resulted in melting points that enabled the formation of films by melt pressing, indicating their thermoplasticity. This study describes the first method to address the problem of preparing thermoplastic chitin derivatives.

This article presented research on the fabrication of chitin-based soft materials through dissolution and gelation in ionic liquids. Although ionic liquids are known as powerful solvents for natural polysaccharides, such as cellulose, only several types of ionic liquid, such as imidazolium carboxylates and AMIMBr, have been found to dissolve chitin. Over the last decade, such ionic liquids have been widely used in facile and practical procedures to produce chitin-based hydrogel, film, and so on. High-DS chitin ester derivatives were produced from chitin by efficient acylation in AMIMBr under suitable conditions, indicating that AMIMBr was a good medium for derivatization. Further, grafting of the thermoplastic polymer, i.e., PCL, allowed to exhibit thermoplasticity on chitin esters. The studies on the fabrication of chitin-based soft materials have notably been developed as described in this article and their medicinal, pharmaceutical, and environmental applications will be further investigated in the future.