
Kenjirou Higashi
Associate Professor, Department of Pharmaceutical Technology, Graduate School of Pharmaceutical Sciences, Chiba University
For over 20 years at the Laboratory of Pharmaceutical Engineering, Faculty of Pharmaceutical Sciences, Chiba University, I have consistently pursued research in molecular pharmaceutics. In recent years, my work has focused on the preparation and structural evaluation of drug-encapsulated nanoparticles. Utilizing cryo-TEM, SAXS/WAXS, solid-state NMR, and molecular simulations, I conduct physicochemical analyses at the molecular level. My research on cyclodextrins has continued since my undergraduate days, and I was honored to receive a Young Scientist Award from a domestic cyclodextrin society.
Pickering emulsions, in which cyclodextrin (CD) inclusion complex crystals precipitate at particle interfaces, have attracted considerable attention. In particular, Pickering emulsions composed of phytosterol ester (PSE) and γ-CD have been shown to be effective for masking capsaicin and controlling drug release. However, previous reports have focused on particles larger than 1 μm, and did not sufficiently investigate nanoparticle formation. In this study, we employed cholesteryl oleate (ChO), the main component of PSE, and optimized preparation conditions to successfully obtain ChO/γ-CD nanoparticles. These nanoparticles exhibited a unique core–shell structure, with a ChO crystal core surrounded by multiple nanosheets of ChO/γ-CD inclusion complex crystals forming the shell. Furthermore, the nanoparticles demonstrated dual-stimulus responsiveness, undergoing structural changes upon heating and exposure to bile components. Because they are prepared from inexpensive, safe, and easily handled raw materials, these nanoparticles hold promise as practical stimulus-responsive carriers for applications across diverse fields.
Cyclodextrins (CDs) are cyclic oligosaccharides composed of glucose units linked by (1→4) glycosidic bonds, and they exhibit excellent biocompatibility 1. CDs possess a bucket-shaped structure and differ depending on the number of glucose units. CDs with 6, 7, and 8 units are referred to as α-, β-, and γ-CD, respectively (Fig. 1a), each with distinct cavity diameters. CDs form inclusion complexes with hydrophobic guest molecules via hydrophobic interactions and van der Waals forces. This property has been widely applied in pharmaceuticals, cosmetics, foods, toiletries, and textiles to mask undesirable taste or odor, improve solubility, and enhance stability against light and heat2.
CDs have also been investigated as dispersants for stabilizing particles3. Recently, Pickering emulsions stabilized by CD inclusion complex crystals have gained attention due to their unique structures, high dispersion stability, and controlled release properties4,5. Sasako et al. reported that micro-sized particles with a core–shell structure can be prepared using phytosterol ester (PSE), a type of lipid6. These PSE/γ-CD microparticles consist of a liquid PSE core coated with a shell of PSE/γ-CD inclusion complex crystals. Encapsulation of capsaicin within these microparticles enables taste masking in the oral cavity and has been implemented in food applications7. Moreover, the microparticles exhibit excellent drug release control, suppressing capsaicin release in the oral and gastric environments while enabling rapid release in the intestinal environment8.
Most reports on CD-based Pickering emulsions describe particles larger than 1 μm. In recent years, various nanoparticles (<1 μm) have been developed for drug delivery. Nanoparticle formation enables efficient drug targeting to tumors and inflamed tissues via injection, and improves drug absorption via oral administration9. To further functionalize PSE/γ-CD microparticles, we investigated nanoparticle formation. Because PSE is a mixture of sterol esters, we employed cholesteryl oleate (ChO), the principal lipid component of PSE, to precisely control nanoparticle properties and elucidate formation mechanisms (Fig. 1b). By optimizing preparation methods and conditions, we successfully obtained ChO/γ-CD nanoparticles10. These ChO/γ-CD nanoparticles exhibited a core–shell structure, with a core composed of ChO crystals coated by multiple nanosheets of ChO/γ-CD inclusion complex crystals. Interestingly, they demonstrated dual-stimulus responsiveness, having undergone structural changes upon heating and exposure to bile components. This paper presents the preparation, structural characterization, and dual-stimulus responsive behavior of these nanoparticles.
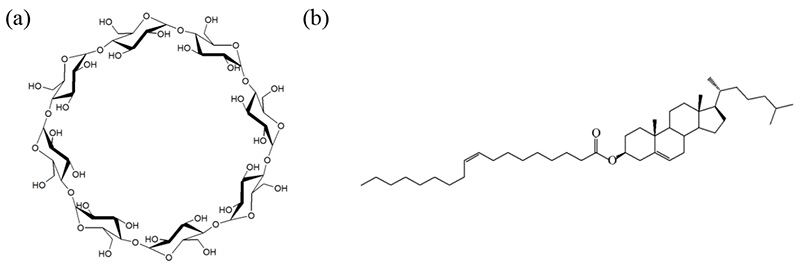
Fig. 2a illustrates the conventional top-down method for preparing PSE/γ-CD microparticles. When PSE, with a melting point of 20–30°C, was homogenized in a γ-CD solution at 70°C, emulsification yielded particles of approximately 5 μm. Attempts to obtain uniform nanoparticles by modifying composition and preparation conditions using this method were unsuccessful. Therefore, we examined a bottom-up approach, specifically the solvent diffusion method. In this procedure, an ethanol solution of ChO was added dropwise into an aqueous γ-CD solution under stirring by a homogenizer (Fig. 2b). This resulted in a nanosuspension (designated NS-I) with a narrow particle size distribution centered around ~170 nm.
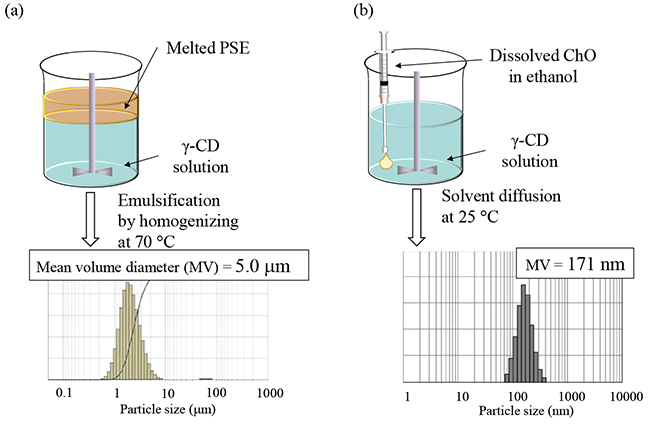
To evaluate the morphology of nanoparticles in NS-I, cryogenic transmission electron microscopy (cryo-TEM) was performed (Fig. 3a)11. Smooth-surfaced particles with diameters of 100–200 nm were observed. However, the expected core–shell structure, in which CD inclusion complex crystals coat the particle surface, was not evident. Subsequent formulation treatments revealed that freezing NS-I induced the formation of particles with a core–shell structure (designated NS-II). Detailed cryo-TEM images of NS-II showed unique structures in which multiple nanosheets—presumed to originate from ChO/γ-CD inclusion complex crystals—coated the particles. The mechanism of nanosheet formation was attributed to freeze-concentration12. Specifically, trace amounts of dissolved ChO/γ-CD inclusion complexes in NS-I were concentrated during freezing together with ChO nanoparticles, leading to precipitation of ChO/γ-CD inclusion complex crystals as nanosheets at the ChO crystal interface.
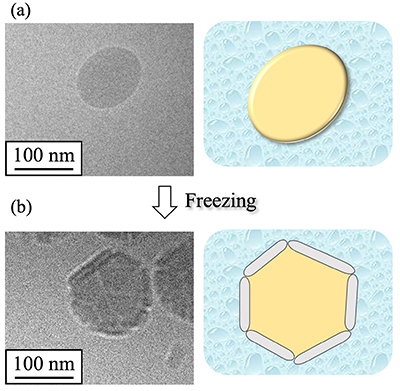
To further characterize NS-II, atomic force microscopy (AFM) was performed (Fig. 4). AFM detects atomic-scale forces between a fine probe tip and the sample, enabling three-dimensional observation of nanoscale structures13. AFM images of NS-II revealed multiple particles with heights of approximately 100–200 nm (Fig. 4a). Force–distance measurements were conducted to evaluate the mechanical properties of the nanoparticles. In these measurements, the probe approaches and retracts from the particle surface while recording the interaction forces. Across 20 particles, the probe did not penetrate the particles, and both the approach curves (Fig. 4b) and retract curves (Fig. 4c) exhibited slopes comparable to those of the substrate. These results indicate that NS-II nanoparticles are relatively inelastic and rigid14.
The structure of NS-II nanoparticles was further analyzed using wide-angle X-ray diffraction (WAXD) (Fig. 5). The WAXD pattern of NS-II (Fig. 5a) displayed diffraction peaks attributable to ChO/γ-CD inclusion complexes (pink squares) and ChO crystals (blue diamonds). This confirmed that NS-II nanoparticles consist of a ChO crystal core and a shell of ChO/γ-CD inclusion complex crystals. For comparison, WAXD patterns of micro-sized ChO/γ-CD inclusion complex crystals prepared by precipitation were examined (Fig. 5b). Both NS-II and the micro-sized crystals exhibited characteristic peaks of the tetragonal-columnar (TC) structure, in which γ-CD molecules are arranged in columnar arrays within a tetragonal lattice of γ-CD15. Interestingly, in NS-II, the diffraction peaks corresponding to crystal planes along the cc-axis of the TC structure showed reduced relative intensity. Because the c-axis corresponds to the length of the γ-CD columnar structure, the lower peak intensity suggests shorter γ-CD columns. This finding reflects the nanosheet morphology observed in cryo-TEM images, indicating that the c-axis direction corresponds to the thickness of the nanosheet shell16.
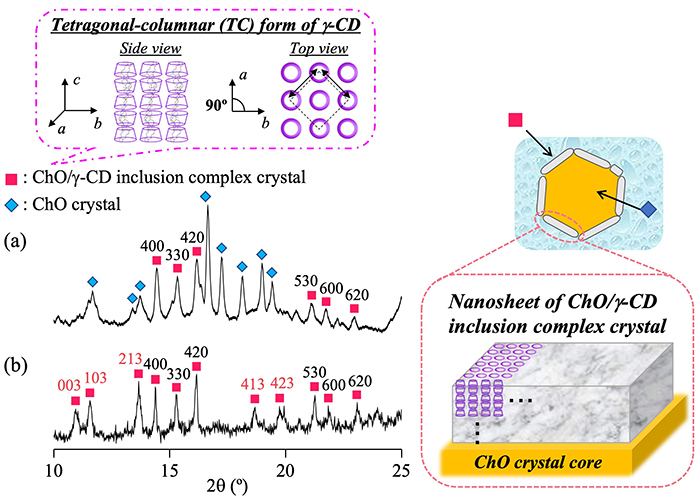
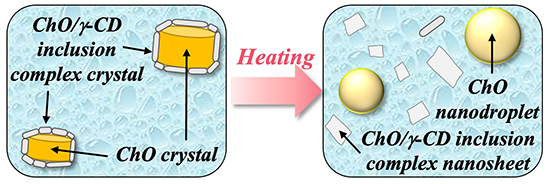
We next evaluated the structural changes of ChO/γ-CD nanoparticles in response to heating by cryo-TEM. The schematic illustration is shown in Fig. 6 After heating NS-II to 60°C, only smooth-surfaced spherical particles and nanosheets were observed, and the core–shell nanoparticles present at 25°C had completely disappeared. This result clearly demonstrates that the nanoparticles lose their core–shell structure upon heating, i.e., the nanoparticle structure is temperature responsive.
To further investigate changes in the core and shell phases upon heating, in situ WAXD measurements were performed on NS-II. Diffraction peaks attributable to ChO crystals decreased markedly in intensity with increasing temperature and disappeared around 45–55°C, indicating that ChO melted within this range to form ChO nanodroplets. In contrast, diffraction peaks from ChO/γ-CD inclusion complex crystals decreased in intensity but remained detectable even at 60°C. This suggests that while some nanosheets dissolved upon heating, their structure was partially retained at 60°C. The observed dissociation of the shell phase was attributed to increased solubility and partial melting of ChO/γ-CD inclusion complex crystals at elevated temperatures, leading to partial aqueous dissolution of inclusion complex crystals at the core–shell interface.
Recent developments in temperature-responsive carriers have primarily relied on phase transitions of thermo-responsive lipids or polymers, with nanoparticle morphology almost maintained17,18. In contrast, the ChO/γ-CD nanoparticles obtained in this study exhibited pronounced morphological changes, with nanosheet dissociation upon heating. This unique behavior positions them as unique temperature-responsive carriers.
We next investigated the effect of bile components on the structure of ChO/γ-CD nanoparticles using fed-state simulated intestinal fluid (FeSSIF)19. Cryo-TEM measurement showed that following bile component addition, cylindrical particles and nanosheets were observed, while the core–shell nanoparticles disappeared. These results clearly demonstrate that ChO/γ-CD nanoparticles undergo bile-responsive structural changes. WAXD measurements of NS-II after FeSSIF addition revealed diffraction peaks attributable to both ChO/γ-CD inclusion complex crystals and ChO crystals, similar to those observed before FeSSIF addition. This confirmed that the cylindrical particles corresponded to ChO crystals, while the nanosheets originated from ChO/γ-CD inclusion complex crystals20.
Fig. 7 illustrates the proposed mechanism of bile-responsive structural changes. Bile acids, the main components of bile components, form micelles in aqueous solution. These micelles solubilize ChO molecules by incorporating them into their hydrophobic interiors21. In addition, bile acids readily form stable inclusion complexes with γ-CD22. In aqueous suspension, trace amounts of poorly soluble ChO/γ-CD inclusion complexes coexist in equilibrium with free γ-CD and ChO molecules. Upon the addition of bile components, ChO solubilization by bile acid micelles and the formation of bile acid/γ-CD inclusion complexes facilitate the dissolution of inclusion complex crystals at the core–shell interface, leading to the dissociation of the shell phase. As a result, cylindrical ChO nanocrystals and nanosheets of ChO/γ-CD inclusion complex crystals are formed.
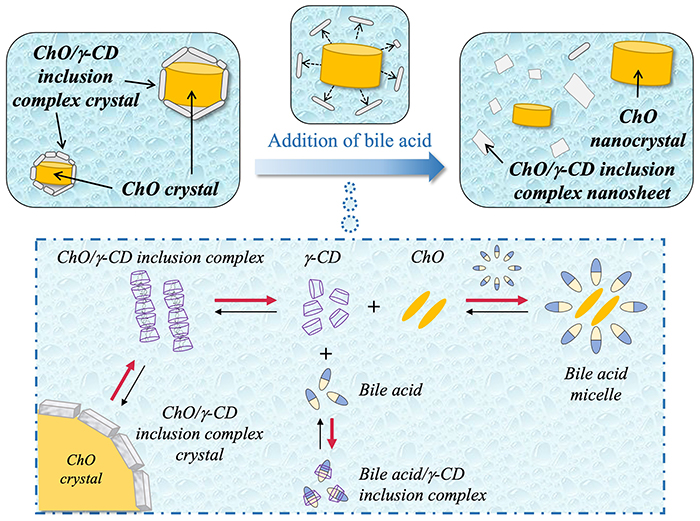
Recent studies have explored various stimulus-responsive carriers, but reports of bile-responsive carriers at the nanoscale level are unprecedented. Because bile acid concentrations are elevated in the intestinal tract, drug release from these nanoparticles is expected to occur after passage through the stomach. Unlike conventional enteric systems that rely on pH-responsive polymers, this mechanism is based on the exchange of guest molecules between bile acids and lipids within CD inclusion complexes. Thus, ChO/γ-CD nanoparticles represent a unique oral enteric formulation strategy.
We successfully prepared ChO/γ-CD nanoparticles with a unique core–shell structure, in which multiple nanosheets of ChO/γ-CD inclusion complex crystals coated ChO crystals. These nanoparticles exhibited distinctive stimulus-responsive properties, i.e., they underwent pronounced structural changes in response to both heat and bile components. Stimulus-responsive carriers for controlled drug release have attracted increasing attention, particularly those responsive to multiple stimuli such as exogenous (heat, magnetic field, light) and endogenous (pH, enzymes) triggers, which enable more precise drug release in vivo23,24. The ChO/γ-CD nanoparticles described here demonstrated dual-stimulus responsiveness: to exogenous heating and endogenous bile components.
Although drug encapsulation within these ChO/γ-CD nanoparticles has already been demonstrated10, detailed investigation of their stimulus-responsive drug release behavior remains to be conducted. Future work will focus on elucidating these release mechanisms and exploring the use of various lipids, CDs, and preparation methods to develop lipid/CD nanoparticles for practical applications in pharmaceuticals, cosmetics, and foods. Because the raw materials used in this system are inexpensive, safe, and easy to handle, the nanoparticles hold strong potential for practical applications.
We sincerely thank Professor Kunikazu Moribe, Assistant Professor Keisuke Ueda, Ms. Arisa Ishimoto, and Mr. Masaki Omori of the Graduate School of Pharmaceutical Sciences, Chiba University, as well as Dr. Kazuo Koyama and Mr. Hiroshi Sasako of House Foods Group Inc., for their invaluable contributions to this study. We also express our deep gratitude to CycloChem Co., Ltd. for providing γ-CD. WAXD measurements in this study were conducted at the synchrotron radiation facility (PF) BL-10C of the High Energy Accelerator Research Organization (KEK) under Proposal Nos. 2019G532, 2021G562, and 2023G596.