
Akira Asari
Dr. Akira Asari is a manager of an hyaluronan oligosaccharides research project of Seikagaku Corporation. He received his Master of Medical Sciences degree from the Graduate School of Medical Sciences, University of Tsukuba, in 1984. He started his study of glycosaminoglycans and proteoglycans as a staff member at Seikagaku Corporation in 1989 after engaging in studies at the Nomura Research Institute of Life Science. He received his doctrate from the University of Tsukuba in 1993. Dr. Asari has been studing cell bological functions of hyaluronan oligosaccharides.
His group found that hyaluronan 4 mers up-regulate expression of heat shock protein 72 thereby suppressing cell death.
Nowadays, many researchers in HA science use hyaluronan (HA) oligosaccharides to study biological and biochemical processes. One of the early studies was reported in 1974 by Hascall and Heinegård 1. Using biochemical methods, they showed that the minimum size of HA that is able to bind strongly to aggrecan is HA-10 mers, (GlcUA-GlcNAc)5. Many HA binding proteins including HA receptors have been found 2, and it is important to elucidate the minimum sizes of HA that bind to them. HA oligosaccharides have also been used to inhibit binding of the HA binding proteins to endogenous HA 3,4 (de la Motte, personal communication).
Noble and his co-workers have shown that HA fragments, that they referred to as low molecular weight HA, induce cytokines, chemokines and so forth that are implicated in inflammation and degradation of extracellular matrix 5,6,7. In addition, there are many reports showing that low molecular weight HA is angiogenic 8, while, high molecular weight HA suppresses angiogenesis 9, production of pro-inflammatory cytokines 10 and of MMPs 11,12, and activation of NF-kappa B 13. Therefore, HA changes biological activities depending on its molecular weight. (Figure 1).
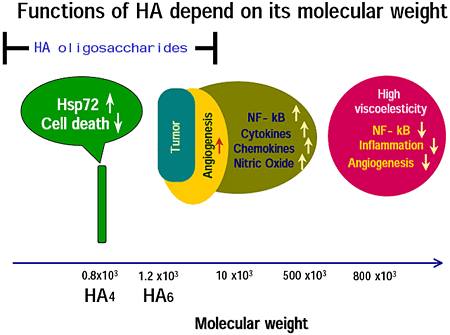
Fig.1
In this article, I refer to HA with molecular weight between 10 kDa and 500 kDa as low molecular weight HA. After studies of low molecular weight HA appeared, HA oligosaccharides entered HA research. Some of the biological activities induced by HA oligosaccharides share those of low molecular weight HA. However, there are many novel activities of HA oligosacharides that are not induced by low molecular weight HA nor by high molecular weight HA. Therefore, HA changes biological activities depending on its molecular weight even though HA consists of simple repeated disaccharides, i.e., GlcA-GlcNAc. This leads to a model for receptors of HA: for example capping induced by high molecular weight HA is not induced by low molecular weight HA. In the case of HA oligosaccharides, one molecule of an HA oligosaccharide can bind to one or two HA receptor(s). For example, one molecule of a 4 or a 6 mer of HA will bind one molecule of a receptor. It is an open question how the binding of one ligand to one receptor induces intracellular signal transduction. Except for the model of HA receptor capping, new models of HA receptor are required for the novel functions of HA oligosaccharides. If the HA receptor has a small, well-like, structure at the binding site, it can bind only to a certain size of HA oligosaccharide but not to higher molecular weight HA (Day AJ, personal communication). Such a receptor is probably specific for a certain size of HA oligosaccharide.
1. Analysis of hyaluronan
Enzymatic depolymerization into disaccharides is required for measuring the quantity of HA by using high performance liquid chromatography (HPLC) or (FACE).
We previously reported precise localization of HA in sections of articular cartilage of human and rabbit tibia by a histochemical technique using a biotinylated HA binding protein 14,15. The measurement of HA disaccharides by HPLC showed zonal differences, i.e., superficial, middle and deep zones in HA concentration, that are well correlated to the histochemical localization.
Disaccharide analyses are viable for measurement of HA content in synovial fluids from arthritis patients. Hydrarthrosis in traumatic arthritis is well correlated with HA content in synovial fluid as determined by HPLC to measure the disaccharides of HA 16.
HPLC analyses for HA require expensive machines. Calabro et al. established the method of fluorophore-assisted carbohydrate electrophoresis (FACE) 17,18. The FACE consists of less laborious procedures without expensive machines. FACE method may progress and become the ordinary method to quatitate HA in the near future.
FACE is also very excellent in evaluation of both the quantity and quality of HA oligosaccharides in a single analysis. HA oligosaccharide libraries have already been analyzed by FACE 19,20.
2. HA receptors and HA binding proteins (Table 1)
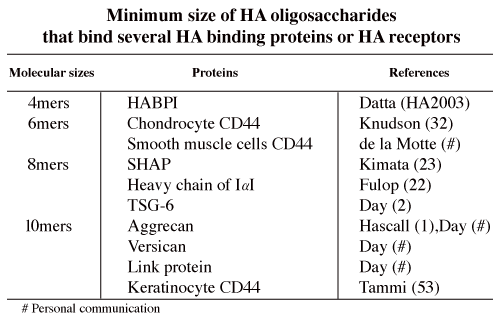
An HA oligosaccharide library is now available to determine the minimum size of HA to bind HA proteins or HA receptors (Table 1), and the differences in signal transductions after binding of several sizes of HA oligosaccharides.
Table 1
HA 4 mers up-regulate Hsp72 expression and suppresse cell death under stress conditions 21. The other sizes of HA do not have such activities. Exogenous HA 4 mers were detected in the cytoplasm of K562 cells (Asari A, personal communication). It is unclear how HA 4 mers can be taken up by the cells. There may be a receptor for HA 4 mers on the cell surface. The K562 cell is known as a CD44-negative cell, but RHAMM mRNA can be detected in K562 cells. However, HA 2, 6 and 8 mers neither up-regulate Hsp72 nor suppress cell death. It seems that we should speculate the presence of a receptor for HA 4 mers that does not recognize other sizes of HA.
Standard type CD44 (CD44s), for example chondrocyte CD44, binds HA 6 mers. On the other hand, the minimum size of HA to bind keratinocyte CD44, i.e., epithelial type of CD44 (CD44E), is 10 mers. CD44 is expressed in many kinds of leukocytes in the immune system. According to their CD44 expression, lymphocytes can be classified into subclasses. de la Motte showed that HA 6 mers but not 4 mers significantly inhibits, a monocytic leukemic cell line (U937s), from binding to HA cables (de la Motte, personal communication). However, little is known about the minimum sizes of HA to bind to other kinds of leukocytes.
Mukhopadhyay et al. found that HA 8 mers and larger oligosaccharides of HA are potent acceptors in the heavy chain (HC) transfer from inter- alupha-trypsin inhibitor (ITI), and thereby inhibit cumulus cell-oocyte complex (COC) expansion 22. Yoneda et al. also showed that HA 8 mers and larger HA are competitive inhibitors for the formation of the SHAP, the HC-HA product formed from ITI 23. In addition, Mukhopadhyay et al. showed that tumor necrosis factor-induced protein 6 (TSG-6) mediates heavy chain transfer from ITI to HA. On the other hand, according to the report of Zhuo, the finding that bikunin is required for the transfer of a heavy chain to hyaluronan uncovers the novel and surprising recognition that bikunin is a “SHAP-presenting” molecule 24. The linkage between HA and SHAP/HC is an ester bond that forms between a C-6 hydroxyl group of an N-acetyl-glucosamine residue of HA and the peptide carboxyl group of an aspartate residue of the HC 25. HA binding proteins other than SHAP/HC bind HA non-covalently. In addition to HA, SHAP/HC, TSG-6 and CD44 of leukocytes, versican interacts with HA to form aggregates similar to those formed by aggrecan. HA oligosaccharides are useful tools for further elucidation of this complex structure in the near future.
The minimum sizes of HA that are bound by the following HA binding proteins (HABP) are not yet definitively elucidated: 1) extracellular HABPs: neurocan, brevican, some kinds of link proteins, spacrcan and SPACR; 2) HA receptors: RHAMM, Lyve-1, LEC/HARE (liver sinusoidal endothelial cell, hyaluronic acid receptor for endocytosis), layilin; 3) intracellular HABPs: cdc37, P-32, RHAMM, IHABP4; 4) Serum HABPs: PHABP (plasma hyaluronan binding protein).
3.HA polymerization
HA oligosaccharides can be used as starting materials or acceptors in HA polymerization by HA synthases (HASs). Addition of unlabeled HA 4 mers derived from testicular hyaluronidase digests to reaction mixtures with recombinant E. coli-derived pmHas stimulated incorporation of radiolabel from UDP-[14C]GlcUA into polymeric hyaluronan by ~20- to 60-fold in comparison to reactions without the oligosaccharide 26.
4. Novel biological activities (Table 2)
When native endogenous HA is degraded in inflammation or tumor tissues, the molecular sizes of the degraded HA are important because low molecular weight HA or HA oligosaccharides can exaggerate the inflammation, cellular infiltration, migration and extracellular matrix degradation through inducing cytokines, chemokines, MMPs, and so forth. Moreover, in inflammation and tumor progression, HA oligosaccharides work as an angiogenic substance. The HA oligosaccharides are one the key components in a vicious circle of inflammation or tumor metastasis.
In sum, HA obtains novel functions or bio-activities after depolymerization into oligosaccharides. Such HA oligosaccharides may bind specific proteins that high molecular weight of HA cannot recognize.
Table 2
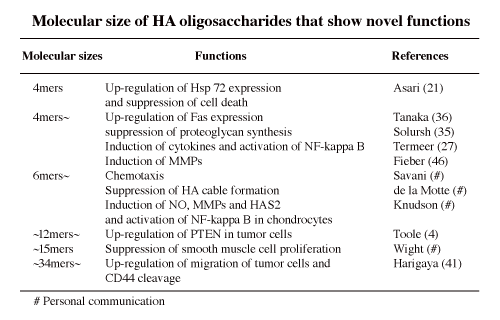
Reports regarding HA oligosaccharides can be classified by 5 viewpoints as described in Table 3.
Table 3
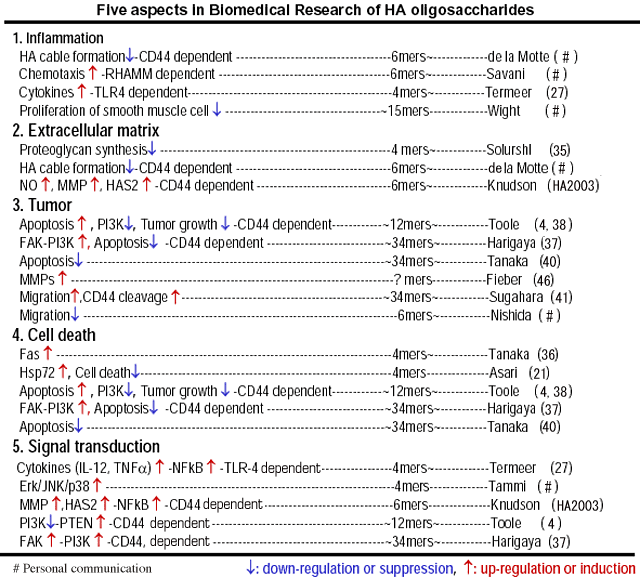
1. Inflammation
Termeer et al. showed that only small HA 4 and 6 mers, but not intermediate size (MW 80, 000-200,000) or high MW HA (MW 1,000,000-600,000) induce immunophenotypic maturation of human monocyte-derived dendritic cells 19. Moreover they showed that oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4 27 . They mentioned that during inflammation, interaction of dendritic cells with small HA oligosaccharides induces dendritic cells maturation. These effects overlap those by low molecular weight HA 5,28,29. The concentration of HA oligosaccharides used mainly in the studies of Termeer et al. was 20 microg/ml. It may be controversial that such a high concentration of HA oligosaccharides is produced or exists at an inflammation site.
de la Motte showed that HA oligosaccharides suppress the binding of U937 monocytes to the HA cables induced by the viral mimetic poly I:C (personal communication). The HA cables induced by poly I:C mimic in vivo extracellular matrices including HA in inflammatory diseases, for example, inflammatory bowel disease 30,31. The minimum size of HA oligossaccharides to suppress binding of U937 cells to HA cables is HA 6 mers (de la Motte, personal communication). This coincides with the result of Knudson et al. who have shown that the HA 6 mer is the minimum size of HA to bind chondrocytes 32. Naïve U937 cells do not bind HA, even though CD44 is expressed on the U937 cells (Asari A, personal communication). However, U937 cells can bind to HA cable structures. In other words, U937 cells prefer HA cables than unsubstituted HA (de la Motte, personal communication). In the presence of SHAP, i.e., heavy chain of ITI, CD44 binding to HA is enhanced (Zhuo L , personal communication). On the other hand, de la Motte showed that an anti-versican antibody that recogniozes G3 of versican inhibits U937 binding to HA cables. These events suggest that versican and/or SHAP initiate(s) the binding of CD44 of U937 cells to the HA. On the other hand, oversulfated chondroitin/dermatan sulfates containing GlcUAbeta1/IdoUAalpha1-3GalNAc(4,6-O-disulfate), that may be contained in versican, interact with L- and P-selectin 33 that may be expressed on the surface of U937 cells.
Savani showed that every size of HA from 6 mers to 900 kDa is chemoattractant for macrophages (personal communication). The chemotactic activity is suppressed by a monoclonal antibody to RHAMM, indicating that the chemotaxis by the HA is dependent on RHAMM. In tumor or inflammatory tissues, HA is degraded into low molecular weight HA or oligosaccharides of HA by free radical and/or hyaluronidases. Such low molecular weight HA is angiogenic 8, and induces cytokines, chemokines and MMPs 28,34. HA oligosaccharides produced in the tumor or inflamed tissues may also be chemotactic to induce inflammatory leukocytes into the tissues. In inflammation the chemotactic activity of the HA oligosaccharides may exaggerate the inflammation.
The novel biological activities found in HA oligosaccharides are implicated in inflammation and degradation of extracellular matrix in many cases. In these cases, contamination of toxins, proteins, nucleic acids and so forth in the HA oligosaccharides samples should be checked, and noted in the report. Quality control of HA oligosaccharides must be done in the preparation of HA oligosaccharides. Contamination into the HA oligosaccharides samples should be avoided, when using HA oligosaccharides in vivo and in cell biological studies in vitro.
2. Extracellular matrices
de la Motte showed that HA cable formation, induced in smooth muscle cells by poly I:C treatment, is also inhibited by the treatment with HA oligosaccharides in a molecular size dependent manner (personal communication). In this case, HA 4 mers do not have the suppressive effect on HA cable formation. HA 6 mers slightly suppress HA cable formation. The HA cables consist of HA, heavy chain of ITI and versican. The minimum sizes of HA binding to the heavy chain or versican is 8 mers and 10 mers, respectively (Anthony J. Day, personal communication). Therefore, it will be elucidated in the near future why 6 mers of HA suppress HA cable formation.
Knudson et al. showed that treatment of cartilage slices with 250 microg/ml of HA 6 mers induces cartilage proteoglycan loss (HA2003)*. They also found that HA 6 mers up-regulate mRNA expressions of matrix metalloproteinase (MMP) 13 and MMP3 by activating retinoic acid receptor (RAR) and retinoid X receptor (RXR), and of type II collagen and cartilage oligomeric matrix protein (COMP) by activating Sp1 in chondrocytes (HA2003)*. This indicates that HA6 mers have dual effects that are implicated in anabolic and catabolic events on extracellular matrices in cartilage. In the near future, it will be elucidated how much of the larger molecular sizes of HA are required, and whether 250 microg/ml or higher concentration of HA6 exist in cartilage tissues from healthy volunteers or arthritis patients.
According to the study of Knusdon et al. described above, HA 4 mers do not induce cartilage proteoglycan loss (HA2003)*. On the other hand, Solursh et al. showed that HA 4 mers and larger sizes of HA oligosaccharides, or high molecular weight HA suppress proteoglycan synthesis in chick embryo chondrocytes 35. This is the first report showing cell biological activity of HA 4 mers. We found that HA 4 mers up-regulate heat shock protein72 21. Tanaka et al. showed that HA 4 mers or larger sizes of HA oligosaccharides enhance Fas expression in synovial cells from rheumatoid arthritis patients 36. In addition, Datta et al. found HABP1 binds 4 mers and larger sizes of HA (HA2003)*. A study regarding activity of HA 2 mers has not been reported yet.
3. Tumor (Table 4)
Harigaya et al. showed that engagement of CD44 by HA (32 kDa) induces tyrosine phosphorylation and activation of focal adhesion kinase (FAK), which then associated with phosphatidylinositol 3-kinase (PI3K) 37. The effect of 32 kDa HA to induce tyrosine phosphorylation is stronger than that of 3,220 kDa or 950 kDa HA. An inhibitor of PI3K, LY294002, induced apoptosis in tumor cells in the presence of etoposide in that study. It was suggested
Table 4
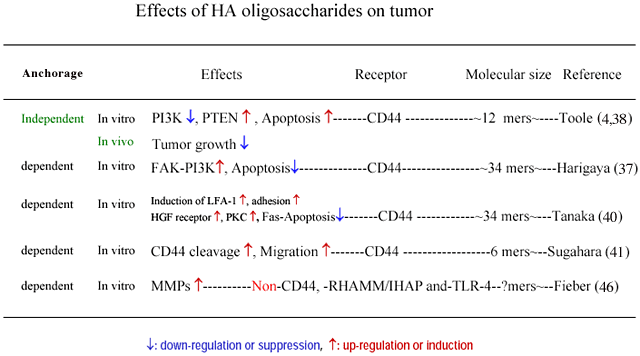
Toole et al. showed that HA oligosaccharides (12 mers) suppress the growth of breast cancer in vivo 39, and also the PI3K induction followed by apoptosis in vitro 4 by perturbing the binding between endogenous HA and tumor cell CD44.
Toole et al. showed that HA oligosaccharides suppress both the MAP kinase and phosphoinositide 3-kinase pathways in multidrug resistant tumor cells and sensitize these cells to a variety of chemotherapeutic drugs 39. They also showed that increased hyaluronan production induces resistance in drug-sensitive tumor cells. This is opposite of the observation of Harigaya et al 37. There is a difference in culture condition between these studies as well as for the cell types and molecular sizes of HA (Table 4). Toole et al. cultured tumor cells in an anchorage independent manner while Harigaya et al. cultured in an anchorage dependent manner (Table 4).
Tanaka et al. showed that treatment with HA fragments down-regulates Fas expression in tumor cells 40. This suggests that HA fragments are implicated in tumor survival by reducing Fas expression.
Sugahara et al. showed that the proteolytic cleavage of CD44 from the cell surface plays a critical role in the migration of tumor cells 41. In that study, HA oligosaccharides (6 mers to 36 mers) enhanced CD44 cleavage by interacting with CD44, whereas high MW HA failed to enhance CD44 cleavage. HA 36 mers not only enhanced CD44 cleavage but also promoted tumor cell motility. In the discussion of the report, it is noted that HA oligosaccharides, which are known to occur in various tumor tissues, promote tumor invasion by enhancing the tumor cell motility that may be driven by CD44 cleavage.
Hyaluronan and hyaluronidase productions, and CD44 expression are progressed in tumor tissues 42,43. Moreover, low molecular weight HA, including HA oligosaccharides, which may be produced by digestion of HA with hyaluronidase exist in tumor tissue 44. It is reported that such low molecular weight HA or HA oligosaccharides are angiogenic 45 and induce cytokines, chemokines 27, and MMPs 46. The MMPs enhance metastasis. This may coincide with the observation of Sugahara et al. The angiogenesis induced by HA oligosaccharides enhances tumor growth. Conversely, the cytokines, and chemokines may function as factors of tumor immunity. Even in this case it is difficult to determine the effects of HA oligosaccharides on tumor progression in vivo. The effects of HA oligosaccharides on tumor growth and invasion may be dependent on the condition of anchorage and/or cell types. Further studies are required to integrate the seemingly controversial results described above.
4. Cell death
Tanaka et al. showed that CD44 cross-linking augmented Fas expression and subsequent Fas-mediated apoptosis of synovial cells from rheumatoid arthritis (RA) patients 36. Hyaluronan fragments also augmented Fas-mediated early apoptosis of the cells. They used HA 34 mers and HA 4 mers as HA fragments in their study. On the other hand, it was reported that the molecular weight of HA in RA patients synovial fluid is reduced 47 and contains such HA fragments. Moreover, apoptosis occurs in synovial lining cells from RA patients 48. Taken together, HA fragments produced in synovial fluids may induce apoptosis by augmenting Fas expression in synovial cells of RA patients.
Conversely, Tanaka et al. showed that treatment with HA fragments that up-regulate Fas expression in RA synovial cells down-regulate Fas expression in tumor cells 40. This indicates that the cytotoxic effect of the Fas ligand of CTL on tumor cells is diminished because of reduction of Fas expression of the tumor cells. In this case, HA fragments are implicated in tumor survival by reducing Fas expression as well as by inducing vascular formation 45.
We found that HA 4 mers up-regulate Hsp72 expression and suppress cell death under stress conditions 21. In that study, treatment of K562 cells with HA 4 mers up-regulated Hsp72 expression after exposure to hyperthermia. On the other hand, treatment of the cells with HA of other sizes (2, 6, 10, 12 mers, 840 kDa), or tetrasaccharides of keratan sulfate did not elicit any change in expression of the Hsp72 protein. Treatment of the cells with HA 4 mers up-regulated not only expression of the Hsp72 protein but also Hsp72 mRNA expression and enhanced activation of HSF1, a transcription factor controlling Hsp72 expression, after exposure to hyperthermia. Because the level of Hsp72 protein was not affected by HA 4 mers when the K562 cells were kept at 37 degrees C without any stress, it is evident that HA 4 mers did not act as a stress factor. In addition, HA 4 mers suppressed cell death in the case of K562 cells exposed to hyperthermia and of PC12 cells under serum deprivation. These results suggest that a certain size of oligosaccharides, i.e., the HA 4 mers, up-regulates Hsp72 expression by enhancing the activation of HSF1 under stress conditions and suppresses cell death.
5. Signal transduction and Induction of proteins
Termeer et al. showed that HA 4 and 6 mers induce immunophenotypic maturation of human monocyte-derived dendritic cells 19 and that oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. 27. The sizes of HA oligosaccharides used in their study were 4-16 mers. Knudson et al. found that HA 6 mers but not 4 mers induce NF-kappa B activation in chondrocytes (personal communication). Termeer et al. also found that NF-kappa B is activated by the treatment with the HA oligiosaccharides 27. Minimum sizes of HA to bind TLR-4 and activate dendritic cells will be elucidated in the near future.
Tammi et al. found that treatment with HA 4 mers activates Erk/JNK/p38 protein expressions in keratinocytes (personal communication).
Moreover, Tool et al. showed that the expression of PI3K, which suppresses cell death, is lowered by the induction of the tensin homolog (PTEN) in the tumor cells by the treatment with the HA oligosaccharides 4. In conclusion, HA oligosaccharides induce cell death (apoptosis) in the tumor cells. The breast cancer cells produce HA that binds CD44 on the cell surface to suppress apoptosis by inducing PI3K. It seems that HA oligosaccharides cancel the endogenous HA signal by perturbing the binding between endogenous HA produced by tumor cells and CD44 on the tumor cells. In addition, the perturbation itself may induce PTEN, otherwise, the HA oligosaccharides may induce PTEN. This may be elucidated by using CD44 positive tumor cells that are not coated with endogenous HA. In the model of the perturbation, the cell surface endogenous HA is replaced by the exogenous HA oligosaccharides. It seems likely that the HA oligosaccharides do not bind the CD44 by detaching the endogenous HA, but bind naïve CD44 that is newly expressed on the cell surface. HA oligosaccharides labeled with a fluorescence reporter are available for examining this possibility. In addition, high molecular weight of HA should be released in the culture media from the tumor cell surface after treatment with the HA oligosaccharides if the oligosaccharides detatch the endogenous HA. Therefore, measurement of the endogenous HA detatching from the tumor cell surface is available for elucidating the precise mechanism of the replacement of HA.
Some HA binding proteins are intracellularly located, while others are found in extracellular matrices. The intracellular HA binding protein, for example HABP1 49, may bind HA in the cytoplasm because HA can exist intracellularly. Such intracellular HA is thought to function in intracellular signal transduction 50, cell division, proliferation 51,52, and so on. Evanko and Wight showed that exogenous HA oligosaccharides penetrate into cytoplasmic reticular structures 52. Exogenous high molecular weight HA is taken-up in vacuolar structures that may be endosomes or phagosomes. The localization of HA oligosaccahrides is different from that of high molecular weight HA in cells, when these exogenous HA are incubated with the cells. This suggests that the exogenous HA oligosaccharides are not involved in degradation pathways, but may be associated with intracellular HA binding protein(s). Moreover, endogenous intracellular HA was found 51. Such HA is probably associated with the intracellular HA binding protein(s). Datta showed that HABP1 binds 4 mers of HA and larger sizes of HA (HA2003)*.
Functions of intracellular HA binding proteins may also be dependent on the molecular sizes of HA. One of the aims of HA research in the next age is to elucidate the function(s) of the intracellular HA binding proteins and HA.