
Warren Knudson
Department of Biochemistry Rush University Medical Center Chicago, IL 60612 USA warren_knudson@rush.edu
Warren Knudson obtained his Ph.D. from the University of Illinois in 1981 working in the laboratory of Dr. Edward Conrad. Following postdoctoral study in Dr. Bryan Toole’s laboratory, where his interest in hyaluronan began, he joined the faculty at Rush Medical College in Chicago in 1985. Currently, he is the Associate Chairman and an endowed Professor of Biochemistry at Rush University Medical Center. Dr. W. Knudson’s area of interest concerns the overall metabolism of hyaluronan. His laboratory has explored the expression and function of hyaluronan synthases, hyaluronidases and the hyaluronan receptor CD44 in articular chondrocytes as well as a variety of model cell lines. Most of the work is aimed at addressing underlying defects in hyaluronan metabolism associated with osteoarthritis. The current focus of the laboratory concerns the internalization and degradation of hyaluronan via CD44, how this mechanism is regulated and how this mechanism coordinates with CD44 signaling, hyaluronan synthase activity and lysosomal hyaluronidase expression. Dr. W. Knudson works closely with Dr. Cheryl
B. Knudson especially in the area of CD44 and cell-matrix interactions.

Cheryl B. Knudson
Department of Biochemistry Rush University Medical Center Chicago, IL 60612 USA cheryl_knudson@rush.edu
Cheryl Knudson received her B.A. degree from Pomona College in Claremont, California and her Ph.D. from the University of Southern California. Her Ph.D. work with Dr. Stephen Meier showed the segmental emigration of cranial neural crest cells and the involvement of mesodermal pattern in their distribution. She also studied the role of hyaluronan in neural crest cell migration, and this directed her interest to postdoctoral studies in the laboratory of Dr. Bryan P. Toole at Tufts University in Boston. With Professor Toole she investigated the role of hyaluronan-cell interactions during limb development, describing the hyaluronan receptor activity expressed during chondrogenesis. In 1985 she joined the faculty at Rush University Medical Center in Chicago and currently is the Ralph and Marian C. Falk Professor of Biochemistry. She is active in the peer review process, directs a summer research program for minority students and is the director of the core curriculum for the Graduate College of Rush University. Her research interests include hyaluronan-cell interactions in the assembly of the pericellular matrix and the regulation of cellular events in the cortical cytoplasm. Her current research focuses on the differential responses by cells to growth factors as dependent on the composition of the extracellular matrix and the interactions between hyaluronan and CD44 during cartilage
differentiation, aging and disease.
One reason hyaluronan attracts considerable attention is its apparent ability to influence cell behavior. This capacity is due in part to the key role of hyaluronan in the organization of a highly-hydrated extracellular matrix and second, the capacity of hyaluronan to interact directly with cells. Probably the most widely expressed and extensively studied hyaluronan receptor is CD44. Although a single gene located on the short arm of human chromosome 11 encodes CD44, multiple mRNA transcripts arise from the alternative splicing of 12 of the 20 exons. Direct splicing of exon 5 to exon 16 generates the standard form of CD44 commonly called CD44H as this is the predominant form on hematopoetic cells. Different combinations of variant exons could potentially yield a myriad of CD44 isoforms. The extracellular domain includes the region primarily responsible for the binding of hyaluronan and the common site for alternative exon usage. The transmembrane domain is highly conserved, and the cytoplasmic domain (exon 20) contains protein motifs with a capacity for interaction with cytoskeletal and signaling proteins. As with other transmembrane glycoprotein receptors, the CD44 isoforms undergo extensive post-translational modifications including glycosylation and phosphorylation. CD44 is at times a hyaluronan receptor - at other times, CD44 participates in signal transduction and rearrangements of the actin cytoskeleton. CD44 serves as a docking protein, as a co-receptor, and can function itself as a nuclear transcription factor. The addition of glycosaminoglycan chains designates that at times, CD44 is an integral membrane proteoglycan. Decisive roles are emerging for CD44 in 1) cell migration during morphogenesis, angiogenesis, and tumor invasion and metastasis; 2) retention, organization and endocytosis of hyaluronan-rich extracellular matrices; 3) mediating the adhesion and rolling of lymphocytes; and 4) coordinating matrix signals that balance cell survival and cell death pathways. This update will review selected advances in CD44 biology, including several structural and functional properties that elucidate how evolution allows utilization of one protein for a wide variety of tasks with small deviations to its structure or in the context of its expression.
A. Alternative splicing between v4 and v5
In our previous review1, the exon usage and alternative splicing related to CD44 mRNA was described. The model of the CD44 gene containing 20 exons, 10 of which are considered variant exons, continues to be the paradigm for CD44 expression in a wide variety of cells. However, this model has been extended in the last few years with the documentation of additional CD44 pre-mRNA splicing variations. One example is the CD44 expressed by synovial cells isolated from the joints of rheumatoid arthritis (RA) patients. These cells can express an mRNA transcript encoding an additional trinucleotide between variant exons 4 and 5 of a CD44v3-10 heparan sulfate proteoglycan isoform2. The authors suggested the name of CD44vRA for the RA-specific CD44v3-10 variant. Transcripts for this sequence arise from the alternative use of intronic sequence between v4 and v5 exons (Fig. 1A). This splicing event resulted in the inclusion of an additional alanine residue without interference in the reading frame. This RA-specific CD44v3-10 variant was detected in fibroblasts from 23 of 30 RA patients analyzed. Cells expressing either CD44v3-10 or CD44vRA bind FGF2 (basic FGF) on the heparan sulfate chains. Upon further examination, the investigators determined that cells transfected with recombinant CD44vRA (or primary RA synovial cells) bound more soluble FGF receptor-1, subsequent to FGF-2 binding, than control cells expressing CD44v3-10 implicating enhanced FGF-R1 activation by cells expressing CD44vRA.
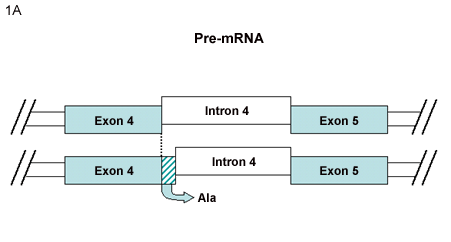
Fig. 1 Alternative Splicing of CD44
A. CD44vRA2 is generated from an mRNA transcript encoding an additional trinucleotide between variant exons 4 and 5. The CD44vRA protein includes an additional alanine residue, with no interference in the reading frame.
B. Alternative splicing between v9 and v10
Yu and Toole3 have described another group of alternatively spliced CD44 isoforms termed “soluble” CD44. This new series of variant CD44 mRNAs was observed in RNA isolated from murine embryonic muscle and cartilage tissues. In addition to CD44v8, CD44v8+9 and, CD44v8-10 isoforms, four new variants of CD44v8-10 were detected containing additional coding sequence from the usage of intronic sequence between variant exon 9 and variant exon 10. Two of these minor transcripts contained extensions of exon 9 (Fig. 1B) while two contained a new small exon (Fig.1C). The authors suggested that what had been previously designated as variant exon 9 (i.e., exon 14) represents only the 5’ half of a larger potential exon. Furthermore, Yu and Toole suggested re-naming the new exon as variant exon 10 (or exon 15) resulting in a register shift of exon numbering of the CD44 gene3. If this nomenclature were utilized, v10 would become v11, and the CD44 gene would include a total of 21 exons instead of 20. A common result of the inclusion of these intronic sequences between the original v9-v10, is that all four of these transcripts contain a stop codon. Thus, translation of any of these four mRNA transcripts would result in a CD44 protein truncated within the membrane-proximal extracellular domain. Upon cloning and expression of these mRNAs, the authors found that the four proteins were synthesized and processed correctly and, as they lack the transmembrane and cytoplasmic domains, the proteins were secreted as “soluble CD44.”
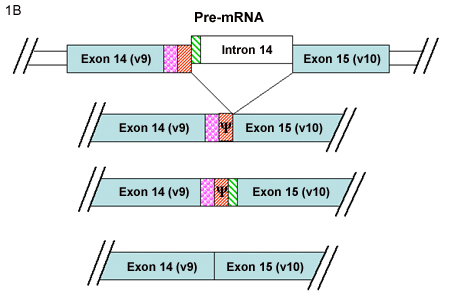
B. Two CD44 mRNA transcripts include extensions of the v9 exon that contain coding sequence from the adjacent intron. These two transcripts contain a stop codon (Ψ); thus upon translation a soluble CD44 protein is generated. The typical splicing of v9 and v10 exons into a CD44 mRNA results in a transmembrane protein with protein extensions within the membrane proximal extracellular domain.
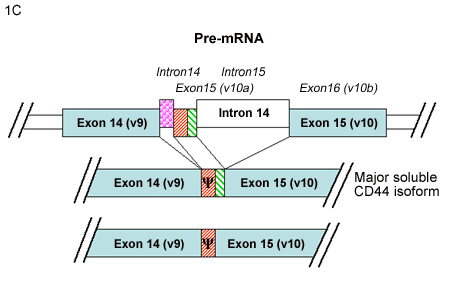
C. Two CD44 mRNA transcripts contain a new small exon, representing coding sequence from the intron previously called intron 14. Yu and Toole3 suggested naming this coding sequence exon 15, which would split intron 14 into intron 14 and intron 15, and results in a register shift of exon numbering. Inclusion of this new exon generates transcripts containing a stop codon (Ψ), and one encoding the major soluble CD44 isoform.
C. Utility of rare CD44 variant isoforms as tools to elucidate CD44 function
The soluble CD44 constructs were put to practical use in subsequent studies. Over-expression and secretion of the soluble CD44 isoforms by various tumor cells interfered with hyaluronan binding to the endogenous CD44 on the surface of these cells4. Thus, the soluble CD44 served as a decoy receptor binding to all available sites on endogenous hyaluronan. Whether such isoforms have a similar decoy function when expressed naturally in cells remains to be determined. Nonetheless, expression of soluble CD44 isoforms provides an important molecular tool to verify that a particular interaction between hyaluronan and the cell surface is dependent on interaction with CD44.
Another CD44 isoform that is generated as a result of alternative splicing also serves as an important tool to validate CD44 function. In our previous review we described the expression of exon 19-containing CD44 mRNA5. Expression of this exon in place of exon 20, would result in the translation of a CD44 with a truncated CD44 cytoplasmic tail domain. Exon 19 contains an early stop codon resulting in a 5 amino acid cytoplasmic tail as opposed to the typical 72 amino acid tail derived from exon 20. Since that time we have found that the recombinant homolog of CD44exon-19 mRNA, pCD44Δ67, lacks the capacity to either bind or internalize hyaluronan6. More interestingly however, when the truncated tail isoform, pCD44Δ67, was co-transfected into COS-7 cells together with a full length human pCD44H, an inhibition of hyaluronan binding function was observed. When pCD44Δ67 was transfected and expressed in primary cultures of bovine articular chondrocytes, the cells lost their capacity to retain a cell-associated matrix6 (Fig. 4G), lost their capacity to bind and internalize hyaluronan7 (Fig. 4F) and, lost their ability to signal via Smad18. Thus, expression of the truncated cytoplasmic domain CD44 isoform (CD44exon19) can serve as a dominant negative receptor, specifically interfering with CD44-mediated interactions with its ligand, hyaluronan. Although a minor natural product of many cells, nevertheless this isoform has usefulness in a recombinant form as a tool to validate putative CD44-related cellular functions.
A. Direct CD44 signaling
Clearly, components of the extracellular matrix can influence cell behavior through interaction with receptor complexes. The direct participation of CD44 in signal transduction remains controversial. Certainly, as a transmembrane matrix receptor with a cytoplasmic domain, CD44 has the basic structure necessary to function, theoretically, in signal transduction. However, the CD44 cytoplasmic tail exhibits no inherent receptor kinase or phosphatase activity. A simple view of CD44-mediated signaling is similar to that of integrin signaling; that is, indirect transfer of extracellular matrix information via associated signaling proteins that become linked to the cytoplasmic domain of CD44. These functional signaling complexes are organized primarily by anchoring proteins and elements of the cytoskeleton. A precise definition of what constitutes the “activation” or the “induction” of the signaling event related to CD44 remains elusive. In nucleated, blood-borne cells, as well as migrating embryonic, endothelial or malignant cells, unoccupied CD44 receptors are “activated” by the binding of hyaluronan, initiating extracellular clustering of CD44 (Fig. 2A and B). The extracellular clustering of CD44 results in intracellular events including the activation of kinases such as c-Src and FAK as well as Rho and Rac9-11, leading to enhanced association of CD44 into actin cytoskeleton complexes and the recruitment and activation of additional signaling partners that lead to cell migration. Lck, Fyn, Lyn and Hck, members of the Src kinase family, co-immunoprecipitate with CD44, and these associations are predominant in lipid rafts12.
Hyaluronan-CD44 interactions also augment the phosphorylation of MAP kinase and Akt in v-Src-transformed cells13. RANTES typically signals via G-protein coupled chemokine receptors. However, its activation of p44/p42 MAP kinase is predominantly mediated by CD44, requiring the initial binding of RANTES to the glycosaminoglycan chains of CD44 variants with such chains14. The use of monoclonal antibody cross-linking to initiate CD44-mediated signaling, such as the activation of Lck and resultant tyrosine phosphorylation of ZAP-7015 or the phosphorylation of Pky212 in T lymphocytes, is additional evidence that the inductive event is the extracellular clustering of CD44. Thus, “outside-in” CD44 signaling can be viewed as extracellular matrix-directed receptor organization that generates changes in CD44 interaction with the actin cytoskeleton and associated signaling protein partners.
In other tissues where hyaluronan is more ubiquitous and in abundance, clustered CD44 receptors occupied in a multivalent fashion with high-molecular-mass hyaluronan represent the quiescent state of the cells. For these cells, CD44 signaling is initiated by disruption of hyaluronan-CD44 interactions (Fig. 2C and D). This disruption may occur by degradation of the hyaluronan16,17, by the presence of soluble CD44 acting as a competitor for hyaluronan4, by cleavage of the ectodomain of CD4418,19 or by the presence and competition of small hyaluronan oligosaccharides20-22. In these instances the release of extracellular constraints or clustering imposed by bound macromolecular hyaluronan is the likely signal induction23.
Multi-faceted apoptotic pathways have been implicated in the pathogenesis of degenerative diseases, and recent data suggest that hyaluronan-CD44 interactions exhibit potent effects on cell survival and sensitivity to apoptosis. Cell survival is mediated in part by maintenance of cell-matrix interactions and directly or indirectly by growth factors (e.g., IGF-1 or BMPs). The addition of hyaluronan oligosaccharides to many cells, including chondrocytes, results in the release of nitric oxide (NO)24,25. This is of interest in that NO itself can down-regulate PI-3 kinase in chondrocytes and induce apoptosis, a process that is reversed by the addition of the cell survival cytokine, IGF-126. Hyaluronan reduces anti-Fas induced apoptosis in chondrocytes27. A hypothesis compiled from these results is that disruption of hyaluronan-CD44 interactions (e.g., stable cell-matrix interactions) would release both CD44 and CD95 clustering, thus priming the cell surface for Fas ligand activation of the apoptotic pathway.
Oligosaccharide-induced apoptosis was also observed in mammary and lung carcinoma cell lines as well as in glioma cells22,28. This also suggests that the loss of hyaluronan-CD44 interactions can result in the induction of apoptosis. Subsequent studies revealed that the induction of apoptosis due to oligosaccharides resulted from an inhibition of PI-3 kinase activity leading to an inhibition of Akt phosphorylation, as well as BAD and FKHR phosphorylation, all of which impinge on Bcl-2 and the activation of caspase-322. In this study, the inhibition of PI-3 kinase was further amplified by hyaluronan oligosaccharide stimulation of PTEN - a phosphatase that dephosphorylates the PI-3 kinase product, phosphatidylinositol-3-phosphate. In addition to hyaluronan oligosaccharides, a similar inhibition of PI-3 kinase activity was also observed by the use of anti-CD44 blocking antibodies. These studies suggest that hyaluronan-CD44 interactions are required for the maintenance of cell survival pathways, a disruption of which may lead to the activation of the apoptotic pathway.
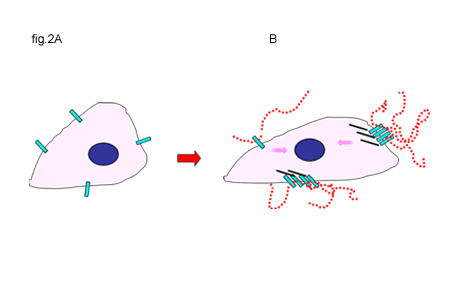
Fig. 2 Activation of CD44 by Hyaluronan Binding or the Disruption of Hyaluronan-Cell Interactions
A and B. In some cells, unoccupied CD44 receptors (A) are activated by the binding of hyaluronan, initiating extracellular clustering of CD44 and the recruitment and activation of additional cytoplasmic signaling partners (B). Signals are represented by the pink arrows.
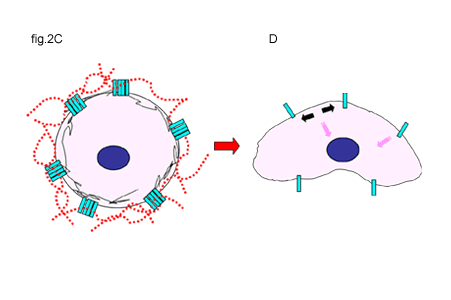
C and D. Clustered CD44 receptors occupied with hyaluronan may represent the quiescent state for other types of cells (C). Disruption of hyaluronan-CD44 interactions releases the clustering of CD44 (black arrows) and is the likely induction of signals (D). Signals are represented by the pink arrows.
B. CD44 and actin adaptor proteins
The association of CD44 with the cytoskeleton ERM (ezrin/radixin/moesin) and/or ankyrin adapter proteins is regulated in part by CD44 phosphorylation29. Morrison et al., proposed a mechanism whereby dynamic associations of CD44 with cytoskeletal complexes could be regulated by changes in the phosphorylation state of ERM proteins and of another adapter protein, merlin30. Merlin (also known as the tumor suppressor gene, NF2) is also a member of the band 4.1 superfamily. Unlike ERM however, merlin has little capacity to engage with the actin cytoskeleton and as such, functions as a competitive inhibitor of ERM-mediated CD44 tethering to the actin cytoskeleton. Phosphorylated ezrin binds CD44, but dephosphorylation of merlin results in its binding to CD44 at the ERM binding site. Thus, the regulated phosphorylation/ dephosphorylation of merlin could function as a regulatory switch by which CD44-hyaluronan interactions impact cell proliferation30. Other investigators have emphasized the importance of band 4.1 proteins in the regulation of ankyrin-CD44 interactions31 and the role of CD44-hyaluronan binding in the formation of intracellular complexes containing ankyrin and other components such as IP3 receptors32.
C. Intracellular domain (ICD) of CD44 as a transactivating factor
Recent reports have documented a new mechanism for direct CD44-mediated signaling: a two step process beginning with an extracellular domain cleavage, followed by γ-secretase cleavage of the transmembrane domain releases the CD44 intracellular domain (ICD), which exhibits nuclear translocation and functions as a transcription factor18,33,34 in a manner similar to the Notch receptor35. The CD44-ICD potentiates transactivation with the p300/CREB-binding protein (CBP) to activate transcription, and one of the target genes identified was CD44 itself18. The ICD release is initiated first by the cleavage of the extracellular domain of CD44 by membrane-associated MMPs36 specifically by MT1-MMP cleavage37 or by ADAM10 cleavage11. This cleavage may regulate, in part, cell detachment from hyaluronan during tumor cell invasion and migration. It is likely that this step may also be facilitated by the docking of MMPs directly to CD44. The two-step MMP/γ-secretase cleavage of CD44 would be expected to modulate CD44s role as a docking protein and as a co-receptor. This dual cleavage pathway also impacts ErbB4, a tyrosine kinase receptor that forms a complex with CD44. Interestingly, treatment of human pancreatic carcinoma cells with small hyaluronan oligosaccharides resulted in the cleavage of the extracellular domain of CD44, presumably via the action of MT1-MMP19, and thus may represent one example of how CD44-ICD generation can be initiated.
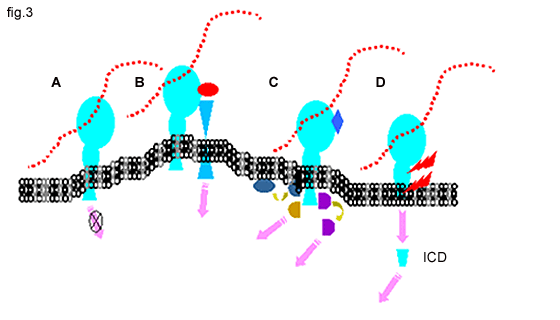
Fig. 3 CD44 in Signaling
A. There is little evidence for direct CD44 signaling upon ligation with hyaluronan.
B. CD44 can function as a co-receptor, physically linked to other classical signaling receptors.
C. CD44 can serve as a docking protein for pericellular proteins, such as MMPs or, for cytoplasmic proteins such as kinases, Smad1, actin-binding proteins and other adapter proteins, which can subsequently activate signal pathways (pink arrows).
D. A two-step proteolytic cleavage (red lightening bolts) releases the CD44 intracellular domain (ICD), which exhibits nuclear translocation and functions in transcriptional activation.
CD44 can also participate in auxiliary functions that are closely related to its role of mediating hyaluronan signal transduction.
A. Function of CD44 as a co-receptor
First, CD44 can serve as a co-receptor, physically linked to other classical signaling receptors such as c-Met38, members of the ErbB family of receptor tyrosine kinases39,40 and TGF-βR141-43, and in the process, facilitate the association of intracellular mediators of signal transduction. Hepatocyte growth factor (HGF) signaling by its cognate receptor c-Met requires the formation of a complex of HGF-c-Met-CD44v6 for c-Met phosphorylation. This interaction with CD44v6 is not due to heparan sulfate substitution. Downstream signaling of c-Met (MEK and Erk phosphorylation) requires the presence of an intact CD44 cytoplasmic domain, whereas the CD44v6Δ67 (truncation mutant lacking the cytoplasmic domain) functions as a dominant negative in the presence of CD4438. In another study, hyaluronan fragments of 3.5 kD (but not macromolecular hyaluronan) stimulated c-Met tyrosine phosphorylation in human chondrosarcoma HCS-2/8 cells44.
B. Function of CD44 as an anchor for matrix metalloproteinases
CD44 can serve as a docking protein for matrix metalloproteinases including MMP-945, MMP-740 and MT1-MMP46. Co-clustering with CD44 retained MMP-9 proteolytic activity on the cell surface, thus promoting type IV collagen degradation and invasion of tumor cells45. Expression of a CD44 truncation mutant lacking the cytoplasmic domain prevented the lamellipodial localization of MT1-MMP, implicating the role of CD44 in linking MT1-MMP to the actin cytoskeleton46.
C. CD44 as a heparan sulfate proteoglycan facilitates signaling
CD44v3 isoforms, bearing a heparan sulfate glycosaminoglycan chain, can provide a support stage for binding basic growth factors such as heparin-binding epidermal growth factor (HB-EGF) and fibroblast growth factors (FGFs)47. Sherman et al.48 presented a model for a novel mechanism for growth factor presentation from the apical ectodermal ridge (aer) cells to the underlying mesenchymal cells in the developing limb bud. In their studies, they determined that CD44 with heparan sulfate on the epithelial cells bound FGF-8 (also synthesized by the aer) and presented it to the signaling FGF-R on the adjacent mesenchymal cells. In another system, Yu et al.40 found that the interaction of the pro-HB-EGF precursor protein with the heparan sulfate proteoglycan CD44v3 facilitates its processing by MMP-7. MMP-7 also docks to the cell surface via CD44v3 and cleaves pro-HB-EGF to generate the active form of the growth factor. Active HB-EGF is then presented to the ErbB4 that is also in a stable complex with CD44. This MMP-7/HB-EFG/ErbB4/CD44 complex participates in the involution of the postpartum uterus and of the lactating mammary gland.
D. Function of CD44 in lymphocyte rolling
Another function of CD44 that is under active investigation is the participation of CD44 in lymphocyte rolling and the infiltration of lymphocytes into sites of inflammation. Prior to forming high strength, integrin-mediated, firm adhesions to the endothelial vessel wall, lymphocytes exhibit a weaker adhesion phase characterized by cell rolling. In rheumatoid arthritis, hyaluronan is elevated in the synovial tissue of joints as well as in the endothelial cell lining of synovial blood vessels. In a proteoglycan-induced animal model of rheumatoid arthritis Mikecz et al., were able to abrogate all swelling and leukocyte infiltration by systemic treatment of the mice with anti-CD44 blocking antibodies49. Antibody treatment resulted in the rapid shedding of CD44 from circulating lymphocytes and synovial cells. Studies by Clark et al. demonstrated that the rolling of tonsillar lymphocytes under shear stress on the surface of cultured human tonsillar stromal cells, was dependent on hyaluronan-CD44 interactions50. Lymphocyte rolling was inhibited by the addition of anti-CD44 monoclonal antibodies, soluble hyaluronan and hyaluronidase treatment. In cutaneous reactions in acute graft-versus-host disease, intense staining for hyaluronan was observed within the dermis, but lymphocyte adhesion was localized to vascular endothelia and not lymphatic endothelia51. Optimal lymphocyte CD44-dermal endothelial hyaluronan interactions also result in lymphocyte homing to these sites of inflammation. These studies verify the long-known concept that hyaluronan-leukocyte interactions were necessary in homing to areas of inflammation52. Pro-inflammatory cytokines, including IL-1 and TNF-α enhance the retention of hyaluronan on the endothelium, thus presenting a surface of immobilized hyaluronan, conducive to lymphocyte rolling. This hyaluronan is anchored to CD44 on the endothelial cells and is resistant to fluid shear forces of the vasculature53. TNF-α would also stimulate the secretion of TSG-6, another hyaluronan-binding protein. Recent studies have shown enhanced lymphocyte rolling and adhesion on TSG-6-hyaluronan complexes compared to hyaluronan alone54. This most likely occurs via the interaction of lymphocyte CD44 with the hyaluronan in the TSG-6-hyaluronan complex, due to either a structural rearrangement of the hyaluronan upon TSG-6 binding or facilitation of CD44 clustering on the lymphocyte. Thus, CD44-hyaluronan interactions play a key role in the initial interaction of lymphocytes with the endothelium and lymphocyte rolling, to enhance leukocyte adhesion at sites of inflammation.
E. Function of CD44 in the internalization of hyaluronan-binding proteins
In our previous review1, we discussed the endocytosis of hyaluronan by cells expressing CD44, such as macrophages and chondrocytes. Fragments of partially-degraded proteoglycan remain bound to hyaluronan and accumulate within tissues with age, and this may contribute to the progression of chronic degenerative diseases. In human articular cartilage, accumulation of link protein and aggrecan N-terminal fragments may block potential binding sites on hyaluronan for the retention within cartilage of newly synthesized aggrecan during periods of repair. In a recent study we determined that chondrocytes have the capacity for CD44-mediated internalization of aggrecan fragments that remain bound to hyaluronan. COS-7 transfectants expressing CD44 gain the capacity for both hyaluronan binding and endocytosis6. Biotinylated aggrecan fragments bound to FITC-hyaluronan were co-internalized in both bovine articular chondrocytes or COS-7 cells transfected with CD44 and were detected within intracellular vesicles that stained with a fluorescent probe for lysosomes7 (Fig. 4A, B, C, D). Intact aggrecan/hyaluronan complexes bound to the cell surface but were not internalized. The internalization of the aggrecan fragments was dependent on the presence of hyaluronan (Fig. 4E and F) as well as the presence of functional CD44. Transfection of articular chondrocytes with CD44Δ67, the cytoplasmic domain truncation mutant isoform, blocked this uptake, suggesting that the over-expression of a CD44 dominant negative mutant in chondrocytes inhibits the binding and internalization of hyaluronan decorated with aggrecan and link protein fragments (Fig. 4G). These results suggest that the capacity for the turnover of proteoglycan fragments bound to hyaluronan may be limited to extracellular matrix in close proximity to cells.
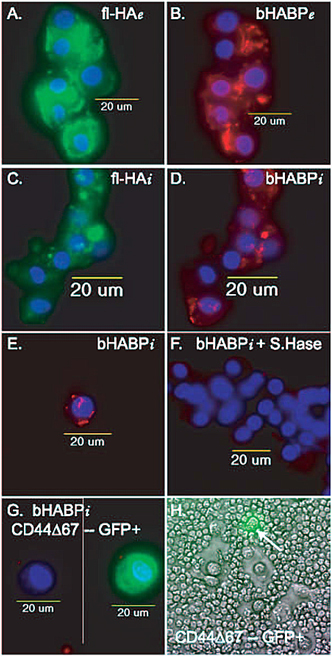
Fig. 4 CD44-Hyaluronan Interactions for Endocytosis and Pericellular Matrix Retention
A and B. Binding of FITC-hyaluronan (fl-HA) decorated with aggrecan fragments (bHABP; Texas Red fluorescence) to bovine articular chondrocytes is visualized as extracellular (e) staining. Nuclei are visualized by the blue DAPI fluorescence.
C and D. Articular chondrocytes incubated with FITC-hyaluronan decorated with aggrecan fragments for 3 hours, were treated with trypsin to remove the extracellular components, revealing the internalization (i) of both fl-HA (panel C) and aggrecan fragments (panel D). Nuclei are visualized by the blue DAPI fluorescence.
E. Internalization (i) of aggrecan fragments (Texas red) bound to hyaluronan by articular chondrocytes.
F.There was no internalization (i) of aggrecan fragments when chondrocytes (blue nuclei) were incubated in the continued presence of dilute Streptomyces hyaluronidase. Thus the internalization of aggrecan fragments is dependent on the presence of hyaluronan.
G. There was no internalization (i) of aggrecan fragments when by transfected chondrocytes over-expressing the dominant negative CD44Δ67 that has a truncated cytoplasmic domain. The cell on the left was visualized in the red and blue channels, with no internalization of bHABP-Texas Red. The same cell, on the right, was visualized in 3-color fluorescence to show the co-expression of GFP indicative of CD44Δ67 expression in the background of the wildtype CD44. Thus the internalization of aggrecan fragments is dependent on the presence of functional CD44. Special thanks to Dr. Jennifer J. Embry of Dr. W. Knudsons laboratory for panels A to G.
H. Retention of the chondrocyte pericellular matrix is dependent on the presence of functional CD44. The pericellular matrix of adult articular chondrocytes was visualized by the particle exclusion assay1. Transfected chondrocytes over-expressing the dominant negative CD44Δ67 (GFP positive cell; arrow) in the background of the wildtype CD44 no longer retain a pericellular matrix. Special thanks to Miss Gabriela Lobato, a high school summer intern in Dr. C. Knudsons laboratory, who performed this experiment.
Assembly and retention of the pericellular matrix of many cells involves CD44-hyaluronan interactions. The CD44 receptor serves as the primary receptor for hyaluronan, providing cells a mechanism for attachment to or for sensing changes in hyaluronan, mediating cell-cell and cell-matrix interactions. CD44 can also serve as a docking protein to organize other molecules in the pericellular matrix or cortical cytoplasm. Recently we reported a mechanism by which changes in hyaluronan-cell interactions modulate the cellular responsiveness to BMPs8. The interaction between CD44 and Smad1 links a BMP signal transduction cascade with CD44-hyaluronan interactions. Functional CD44 expression and CD44-hyaluronan ligation promotes a robust cellular response to BMP-7 that is initiated by Smad1 phosphorylation and nuclear translocation8. Disruption of hyaluronan binding to CD44 results in a decreased BMP response. These studies support the paradigm wherein changes in the hyaluronan-dependent extracellular matrices modulate signal transduction, culminating in differentiation, disease or tissue homeostasis.