
Takuya Sagawa
Assistant Professor, Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science
In 2018, he received his Ph.D. in Engineering from Tokyo University of Science under the guidance of Prof. Takahiro Gunji and joined the group of Prof. Atsushi Fukuoka at Hokkaido University as a postdoctoral researcher. In 2020, he moved to the group of Prof. Mineo Hashizume at Tokyo University of Science and has been in this post ever since. His research interests are the fabrication and evaluation of composite materials composed of polysaccharides, and the conversion of chitin-derived amide sugar alcohol.

Mineo Hashizume
Professor, Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science.
He received his Ph.D. in 1999 from Tokyo Institute of Technology. After one year of postdoctoral research experience at the University of Texas at Austin, he joined RIKEN as a frontier researcher. In 2002, he moved to Nara Institute of Science and Technology as an Assistant Professor. In 2008, he became a junior associate professor and started his own research group at Tokyo University of Science. He was promoted from associate professor to professor in 2017. His research interests include utilization of polysaccharides as functional materials and creation of organic-inorganic hybrid materials using biomimetic processes.
Chitosan is obtainable from chitin, an abundant polysaccharide, and has high biocompatibility and biodegradability. Therefore, chitosan can be used as biomaterials. Chitosan is soluble in acidic aqueous solutions and acts as a polycation. In this article, we describe a method for utilizing chitosan as structural materials (i.e., polysaccharide composite films obtained by molding polyion complexes that are formed by mixing chitosan and anionic polysaccharides). The polysaccharide composite films possess functional features such as the ability to load drugs, absorb moisture, and retain moisture, indicating that they could be useful as biomaterials in applications such as drug sustained-release carriers and wound dressings.
Chitosan (CHI) is generally obtained from deacetylation of chitin, which is the most abundant biomass in the ocean (Figure 1A), and directly from some kinds of fungi. Both chitin and CHI possess high biocompatibility, biodegradability, and low toxicity. Therefore, they can be utilized as structural biomaterial in applications such as wound dressings and drug sustained-release carriers1,2. Chitin is a water-insoluble polysaccharide, which has been reported to have antibacterial and wound healing effects. The water insolubility of chitin is derived from its rigid crystal structure, which has been attributed to three-dimensional hydrogen bonding3, and it is often used in biomaterials that require high strength, such as biomaterials used in wound dressings4. However, chitin has poor moldability, and thus it is difficult to handle during material production. On the other hand, CHI is easier to handle and mold than water-insoluble polysaccharides due to its solubility in acidic aqueous solutions. CHI is composed of ᴅ-glucosamine, which has an amino group at the 2-position. In acidic aqueous solutions, this amino group is protonated and becomes an ammonium cation, resulting in the increase of solubility of CHI in water. A polymer including a large number of charges in an aqueous solution is called a polyion or polyelectrolyte, and a polymer having a large number of cations or anions is called a polycation or polyanion, respectively. CHI is the only polycationic polysaccharide in nature, and therefore it has been actively researched as a cationic polysaccharide1,4,5.
It is relatively easy to mold CHI in the dissolved or dispersed state into a desired shape, but when the molded materials are used in aqueous environments, swelling makes their shapes difficult to maintain. Notably, they dissolve in acidic environments. Therefore, crosslinking agents are often used to make the material water-insoluble as in the case of CHI used as structural materials in biomaterial applications2,6,7. However, the use of crosslinking agents may reduce CHI’s antibacterial properties and biocompatibility.
One of the methods for insolubilizing water-soluble polysaccharides is the formation of polyelectrolyte complexes (polyion complexes, PICs). Not limited to CHI, cationic polyelectrolytes can interact with anionic molecules and inorganic ions that have the opposite charges. This property may work advantageously when fabricating bulky, gel-like, film-like, and fiber-like structural materials from polyelectrolytes. PICs are polymer complexes formed by mixing polyelectrolytes with opposite charges, and they are insoluble in various solvents including water due to formation of three-dimensional crosslinks through electrostatic interaction. A polycation in aqueous solution, CHI forms complexes with many kinds of polyanions, including polysaccharides such as chondroitin sulfate C (CS) and hyaluronic acid (HYA) (Figure 1A). When these oppositely charged polyelectrolytes are mixed, water-insoluble PICs are formed (Figure 1B)8-11. One advantage of this method is that no cross-linking agents or chemical modifications are required to produce water-insoluble gels. Therefore, it is useful because PICs can be molded into materials while retaining the intrinsic physical and chemical properties and functions of the polysaccharides. Furthermore, the degree of swelling and elastic modulus of PICs composed of polysaccharides can be modulated by changing the polysaccharide species, the mixing ratio of polysaccharides, and the pH of the solution.
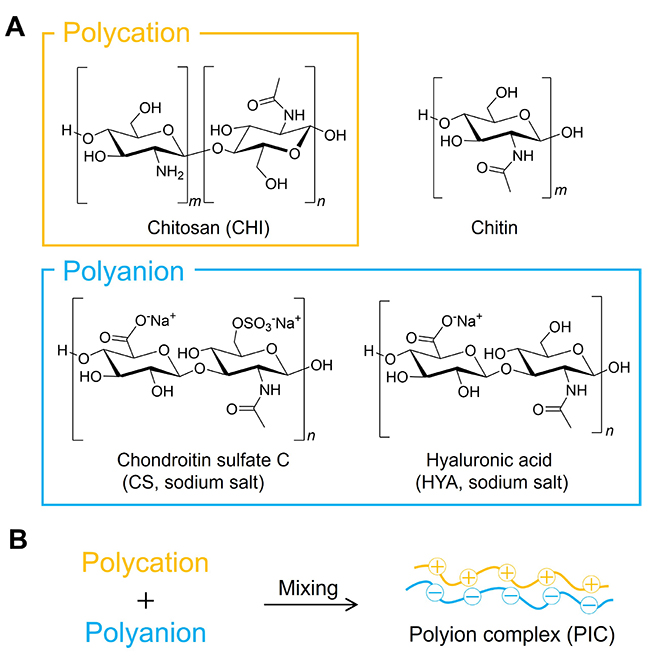
Film materials consisting of polysaccharides are useful as two-dimensional structural materials, and they are utilized as biomaterials in applications such as two-dimensional scaffolds and wound dressings. The films of PICs composed of polysaccharides are often prepared by solution casting or layer-by-layer (LbL) assembling techniques2,12-14. In the case of solution casting, the preparation of homogeneous films is difficult. In the case of LbL assembly, alternately-assembled films of CHI and anionic polymers are obtained. The thickness of the films can be controlled by the number of cycles, but it takes a longer time to obtain thicker films. Also, to use the laminated layers as films, it is necessary to detach them from the support solid substrates. Films having thicknesses of several tens of nanometers are often prepared, but they are not free-standing and their handling is difficult. On the other hand, we have established a method to prepare films with thicknesses of several tens of micrometers reproducibly by molding PIC gels that obtained by mixing CHI and anionic polysaccharides using hot press techniques8,9,15,16. This method is facile and the obtained films (hereinafter called “polysaccharide composite films”) are sufficiently free-standing to be easily handled manually.
As an example, the preparation of polysaccharide composite films consisting of CHI and CS (CS/CHI films) is briefly described (Figure 2, upper row)8,9. A CS aqueous solution was mixed with a CHI acetic acid solution, and the mixture was vigorously shaken to promote PIC formation. Additional CS solution was added dropwise to the mixture until no further PICs were formed, and the obtained gel-like PICs were isolated by centrifugation. After that, hot-pressing was performed using a hot-press apparatus (AH-2003 or H300-15, AS ONE Corp.). The PIC gels were sandwiched between two polytetrafluoroethylene (PTFE) sheets, and polyethylene terephthalate (PET) sheets (100 μm thickness) were placed above and below. The water in the gel was evaporated by placing the sample in the apparatus for 10 seconds, in contact with the upper and lower stainless-steel plates, which were heated to 120°C. Then, the pressed gel was folded into a small piece, placed in the cut-out center of a PET sheet (100 μm thickness) used as a spacer, which was sandwiched between two PTFE sheets, and subjected to hot-press molding at 20 MPa for 3 minutes to form the CS/CHI film. The films obtained by this method had thicknesses of about 80 μm and were colored slightly yellow. This color was probably due to the progress of the Maillard reaction and the formation of humic acids by heating17. This coloration can be avoided by preparing films at a lower hot-press temperature of 35 °C16. Furthermore, the same techniques have been successfully used to prepare composite films of CHI and other anionic polysaccharides such as HYA9,18. Macroscopic and microscopic morphologies of those films were similar to the macroscopic and microscopic morphologies of CS/CHI films. As described above, the present methods (hot-press techniques) are highly versatile for producing polysaccharide composite films with dense structure and high film formability. These polysaccharide composite films exhibited high mechanical strength based on their dense structure. Especially, the CS/CHI films show the maximum stress value of approximately 80 MPa. Further, when immersed in water, the films swelled quickly and then the swelling ratio remained constant after 30 min. That is, the films are basically water-insoluble. Though swollen films are soft with decreased mechanical strength and free-standing properties, they can be improved by using support membranes or adding fillers15,19. To explain the swelling property in more detail, the swelling ratio depends on the polysaccharide species and the pH and ionic strength of the solution. For example, some kinds of films gradually decompose after prolonged incubation at 37°C in phosphate-buffered saline (PBS). In other words, the polysaccharide composite films can be treated as biodegradable films depending on the conditions.
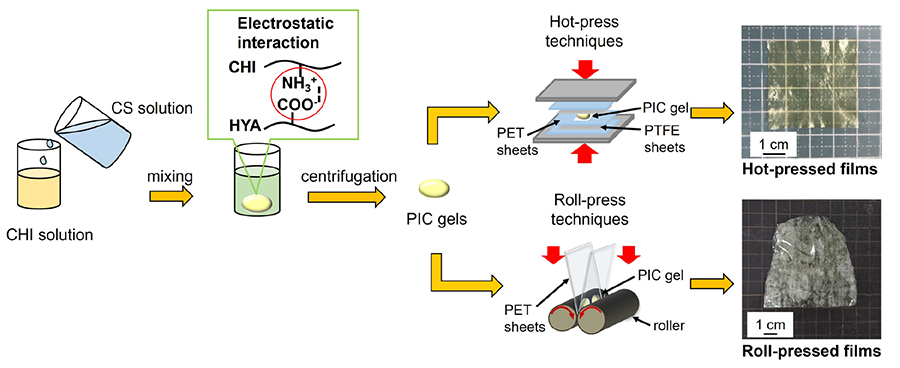
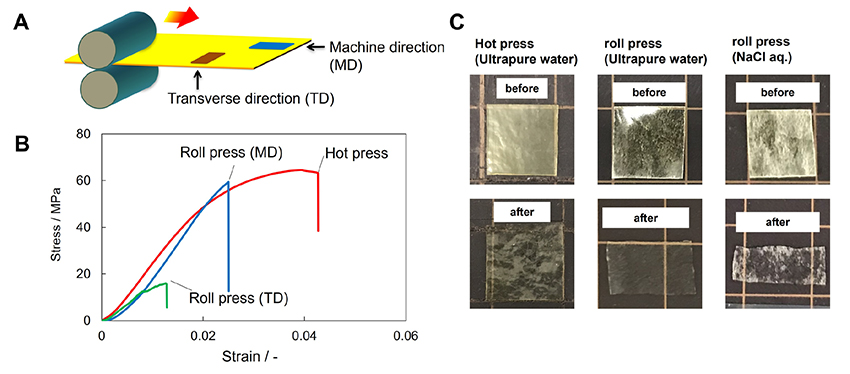
Polysaccharide composite films can be formed not only by hot-pressing but also by heat stretching (Figure 2, lower row)20. For PIC gels consisting of CHI and CS prepared as described above, heat-stretching was conducted using a heated roll-press apparatus (IMC-1107, Imoto Machinery Co., Ltd.). The PIC gels were sandwiched between 100 μm thick PET sheets and rolled between rollers (40 mmφ). After that, the gels were folded and rolled several times to produce heat-stretched films. The resulting films were transparent, flexible, and free-standing like the hot-pressed films. An investigation to determine optimal heat stretching conditions confirmed that the roll temperature and rolling speed have a large effect on the formability of the film. In particular, the roll speed at 8 rpm and the roll temperature at 120 °C gave the highest film formability. Tensile tests of heat-stretched films revealed that the mechanical strength of the films in the machine direction (MD) was approximately five times higher than that in the transverse direction (TD), confirming that heat stretching imparted mechanical anisotropy to the films (Figure 3A and B). Furthermore, the film shrank in the MD direction and expanded in the TD direction when immersed in an aqueous solution and then dried (Figure 3C). The shrinkage and expansion indicate that heat-stretched films can be used as shape memory materials. In addition to films, polysaccharide PIC can be formed into fibers using interfacial spinning21.
A functional evaluation of these polysaccharide composite films has also been performed. For example, to evaluate the potential application of the films as a biomaterial such as a wound dressing, we investigated cell culture on the films made of CHI and various anionic polysaccharides22. The results showed that these films had low cytotoxicity, and cell activity could be controlled by the anionic polysaccharide species. In addition, investigations of the usefulness of the films as selectively permeable membranes are in progress23.
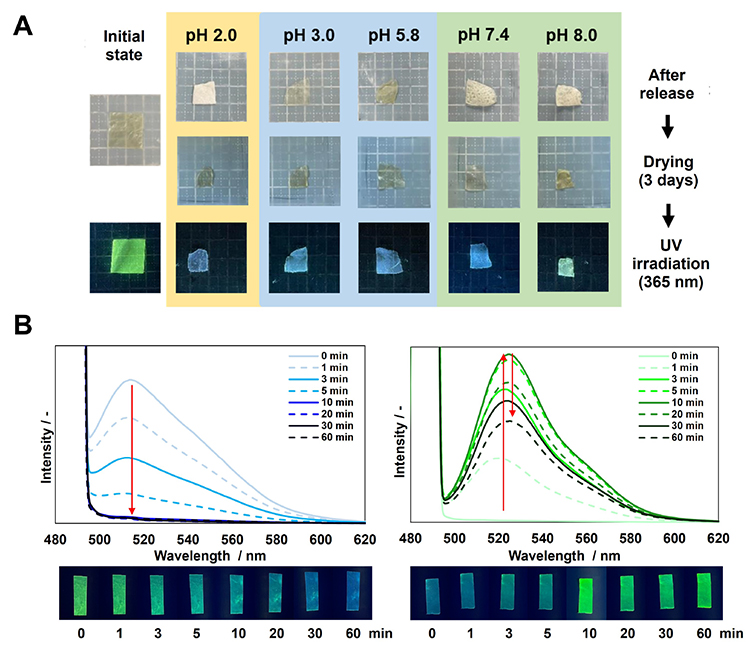
Here, we describe the molecular loading ability of the polysaccharide composite films. CS/CHI films can load and release drug model molecules, and the release rate of the molecules changes depending on the pH environment24. Furthermore, in order to clarify the pH responsiveness and the interaction with polysaccharides of the molecules loaded in the films, fluorescein (FL)-loaded CS/CHI films were prepared and the ionization and release behavior of FL were evaluated16. The FL-loaded films were prepared by adding FL to the solution used for PIC formation and by using hot-press techniques. The resulting films were pale yellow and exhibited FL-derived fluorescence upon irradiation with 365 nm UV light (Figure 4A). When the films were immersed in phosphate buffers (pH 2.0, 3.0, 5.8, 7.4, 8.0), the release behavior of FL was different depending on the pH of the solution, the electrostatic interaction between polysaccharides, and the ionic structure of FL. However, more than half of the FL molecules still remained in the films even after the release experiment. All of the films emitted FL-based fluorescence upon irradiation with 365 nm UV light (Figure 4A). Here, it was confirmed that the ionization state of FL remaining in the films after immersion in buffered solutions at each pH was different from that in solution. The difference was thought to be caused by the electrostatic interactions and hydrogen bond formation between the CHI and CS in the film and the FL in the film. Moreover, when the pH responsiveness of FL in the dried films under a hydrogen chloride and ammonia atmosphere was evaluated, it was found that the ionic structure of FL in the film changed in both cases (Figure 4B). These results indicate that the ionic state of the loaded molecules can be controlled within the film, and it is also possible to fix molecules within the film in a specific ionization state. Furthermore, these films can load molecules with surrounding three-dimensionally by CHI and CS depending on the shape and charge state of the molecules. Thus, they can load various kinds of molecules. These properties suggest that these films can be used as pH-responsive materials in applications such as drug sustained-release carriers.
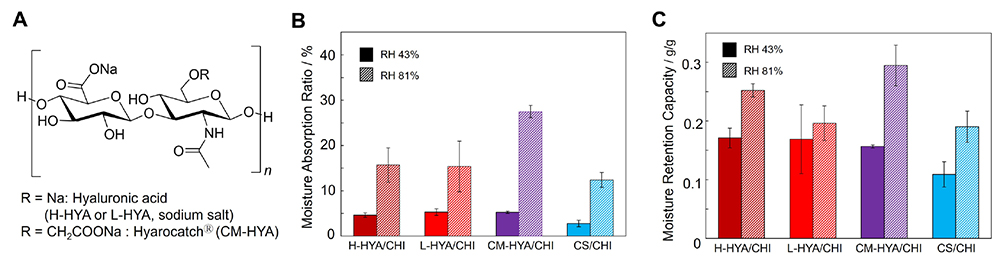
Materials composed of polysaccharide PICs have the advantage that they can be molded without losing the properties of the polysaccharides. HYA has excellent biocompatibility and biodegradability, and it promotes cell adhesion, migration, and differentiation. It is also involved in many cellular processes, including wound healing25. Furthermore, HYA has excellent moisturizing ability, viscoelasticity, and lubrication properties, so it is expected to be a suitable material for wound dressings. Herein, polysaccharide composite films made of PICs of CHI and HYA (hereinafter designated HYA/CHI films) were prepared, and the effects of the molecular weight and chemical modification of HYA on its physical properties in the swollen state were investigated18. Specifically, high molecular weight HYA (H-HYA, molecular weight 1,200,000 to 2,200,000), low molecular weight HYA (L-HYA, molecular weight ≥ 10,000), and an HYA derivative with carboxymethyl groups linked to the primary hydroxyl groups of HYA (CM-HYA, molecular weight 800,000 to 1,200,000, modification rate 65 to 95%) were prepared (Figure 5A). All of the obtained films were colored pale yellow and flexible. The mechanical strength of each film was measured by tensile tests, and CM-HYA/CHI (98.8 ± 4.1 MPa) showed higher strength than H-HYA/CHI (81.3 ± 5.9 MPa) and L-HYA/CHI (70.2 ± 5.9 MPa). The higher strength was due to an increase in the amount of carboxylic acid groups and ionic crosslink density in the film. HYA has one carboxy group per disaccharide repeating unit, while CM-HYA has 1.65–1.95 carboxy groups. In addition, the swelling ratio of the films in ultrapure water and PBS (pH 7.4) was confirmed. In ultrapure water, the swelling ratio increased in the order of L-HYA/CHI > H-HYA/CHI > CM-HYA/CHI. On the other hand, the swelling ratio of L-HYA/CHI decreased with time in PBS, indicating that the L-HYA in the film had dissolved into the solution. When comparing HYA/CHI films with similar molecular weight of HYA, CM-HYA/CHI had a lower swelling ratio than H-HYA/CHI. From the above results, it can be concluded that the CM-HYA/CHI films showed the highest maximum stress value and the lowest degree of swelling among the other prepared films, indicating that CM-HYA improves the physical properties of polysaccharide composite films. Moreover, the moisture absorption and moisture retention ability of each film were also investigated. The moisture adsorption of the films was determined by measuring the weight change after drying in a desiccator at a relative humidity of 43% and 81% for 48 hours. The moisture retention was determined by measuring the weight change of films swollen by soaking in PBS (pH 7.4) and then put in a desiccator at a relative humidity of 43% and 81% for 48 hours. The CM-HYA/CHI films showed the highest moisture adsorption and moisture retention compared to other HYA/CHI films and CS/CHI films (Figure 5B and C). These results demonstrate the influence of polysaccharide type, molecular weight, and charge density on the physical properties of polysaccharide composite films. Moreover, polysaccharide composite films made of CHI and CM-HYA can be used as biomaterials in applications such as wound dressings and cell scaffolds because of their high hygroscopicity and moisture retention properties.
Polysaccharide composite films with good handling properties were prepared from PICs consisting of CHI and anionic polysaccharides, and their physical properties and functions were evaluated. The polysaccharide composite films developed by us are easy to mold because they are obtained from water-soluble polysaccharides, and they can be expected to be used in a variety of structural materials. The formation of film structures is driven by the formation of non-covalent bonds, and it is possible to prepare composite films containing molecules of various sizes and charges in addition to CHI and anionic polysaccharides. By changing the species and molecular weight of the polysaccharide, it is possible to control the physical properties of the films such as swelling, moisture adsorption, and moisture retention. These properties support the notion that polysaccharide composite films are useful biomaterials in applications such as drug sustained release carriers and wound dressings. There is a wide variety of anionic polyelectrolytes that can be complexed with CHI, not just polysaccharides, and it is expected that this approach will further expand the potential applications of CHI as a material.