
Makoto Ogata
Associate Professor, Faculty of Food and Agricultural Sciences, Fukushima University, Ph.D. (Agriculture)
In 2008, he completed his doctoral course in the Department of Sciences of Bioresource Chemistry at the United Graduate School of Agricultural Sciences, Gifu University, and obtained his Ph.D. (Agriculture) in 2008 (Gifu University). After a post-doctoral training period, he was appointed as an assistant professor at the National Institute of Technology, Fukushima College, in 2012, an associate professor at the National Institute of Technology, Fukushima College, in 2015, and an associate professor at Fukushima University in 2020. He is engaged in research into the functional design of glycan-conjugated molecules based on chemoenzymatic synthesis.
Awards: Japanese Society for Chitin and Chitosan Encouragement Award (2024), The Japanese Society of Applied Glycoscience Encouragement Award (2021), The Japanese Society of Applied Glycoscience Technology Development Award (2020), Japan Society for Bioscience, Biotechnology, and Agrochemistry (JSBBA) Encouragement Award (2020), Intelligent Cosmos Encouragement Award (2017), etc.
Chitin is a promising biomass resource, and an estimated 100 billion tons are produced annually on Earth by chitin-biosynthesizing organisms, such as crabs, shrimp, squid, rhinoceros beetles, crickets, and shiitake mushrooms. Its structure is a linear chain of N-acetylglucosamine (GlcNAc) linked via β-1,4-glycosidic bonds that form numerous intra- and intermolecular hydrogen bonds, resulting in a highly crystalline fiber structure. Currently, research on biorefinery processes utilizing the structural properties and biofunctions of chitin is being actively conducted by many researchers from the environmental, resource, and energy issue perspective in a wide range of fields, including molding, veterinary and pharmaceutical materials, biotechnology, cosmetics, food, agriculture, forestry, fisheries, and industry1. However, considering the potential of chitin as biomass, its utilization is currently insufficient. This is because chitin has a lower solubility in common organic solvents compared to cellulose, a woody biomass, which reduces its processability and usability. As part of our research on chitin biorefinery, our group is focusing on chitin decomposition products, which are more soluble in water than chitin and have significantly improved processing properties. Chitin decomposition products are mixtures of GlcNAc and chitin oligosaccharides (degree of polymerization 2 to 6) that are industrially produced from chitin. In this article, we introduce the part of our research focused on the utilization of chitin decomposition products as biomass resources: our studies on the reconstruction of in vivo glycans and the creation of biofunctional molecules.
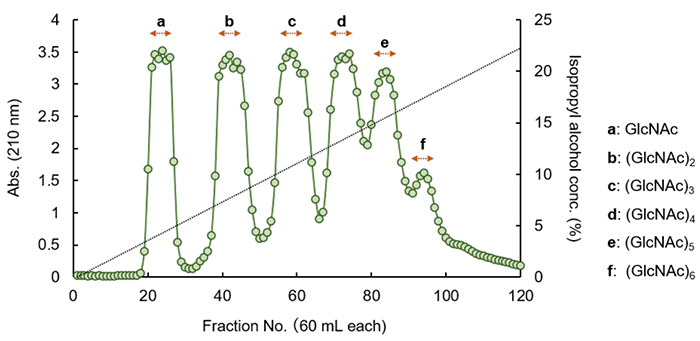
Chitin decomposition products are mixtures of GlcNAc and chitin oligosaccharides, and further isolation and purification processes are required to use them as raw materials for precise material development. Of course, GlcNAc and chitin oligosaccharides prepared with a single degree of polymerization can be purchased as general reagents, but chitin oligosaccharides, even N-acetylchitobiose, a disaccharide, are very expensive. While realizing that each laboratory may have its own secrets and procedures for the purification of monosaccharides and oligosaccharides from chitin decomposition products, we would like to take this opportunity to introduce our research group's method. Here, we show an example using 5 g of chitin decomposition products manufactured by Tokyo Chemical Industry Co., Ltd. (TCI). To purify GlcNAc and chitin oligosaccharides from chitin decomposition products, we used a charcoal-Celite column (4.5 cm in diameter, 40 cm in height), which was prepared using a slurry of activated carbon powder and Celite in equal amounts. This method utilizes the property of monosaccharide and oligosaccharide binding affinity to activated carbon powder, which differ depending on the degree of polymerization, and Celite, which acts as a filtering aid. Elution was performed with a 10 L linear gradient of 0→31% isopropyl alcohol, and fractions of each degree of polymerization were obtained by sequentially eluting GlcNAc to (GlcNAc)6 contained in the chitin decomposition products (Figure 1). The recovered fractions were analyzed by high performance liquid chromatography (HPLC), and the results were as follows: GlcNAc, 1.5 g; (GlcNAc)2, ca. 0.8 g; (GlcNAc)3, ca. 0.8 g; (GlcNAc)4, 0.5 g; (GlcNAc)5, 0.3 g; (GlcNAc)6, 0.1 g. Furthermore, HPLC analysis showed that the purity of the GlcNAc to (GlcNAc)5 fraction was 96-99%, and that of the (GlcNAc)6 fraction was less than 60%. As a bioactive oligosaccharide that has been found to stimulate plant growth and animal immunity, (GlcNAc)6 can be further purified by gel filtration chromatography using Bio-Gel P2 or the like to obtain a purity of 98% or higher. As described above, from several hundred milligrams up to a gram of GlcNAc and chitin oligosaccharides, prepared with a single degree of polymerization, can be obtained from chitin decomposition products by a simple one-step column separation procedure.
In the latter half of the 20th century, advances in the field of glycobiology have shed light on the structure and biosynthesis of in vivo glycans, and their relationship to biological phenomena2. The functions of glycans are extremely diverse, ranging from glycan-specific functions, such as those involved in the supply of structural components and modification of the physical properties of proteins, to glycan-lectin interaction-specific functions, such as those involved in the transport management of glycoconjugates and the mediation and regulation of cell adhesion and signal transduction2. Soon after, information obtained from glycobiology research led to the field of glycotechnology and more particularly to the artificial creation of various sugar substances that contribute to life sciences3. However, there are many challenges to isolate and purify various biologically active glycans that exist in very small amounts in nature and living organisms, and apply these biologically active glycans to the development of engineered materials. Therefore, a method to reconstruct biologically active glycans in a bottom-up manner by combining inexpensive carbohydrates was devised for the preparation of functional glycan libraries4.
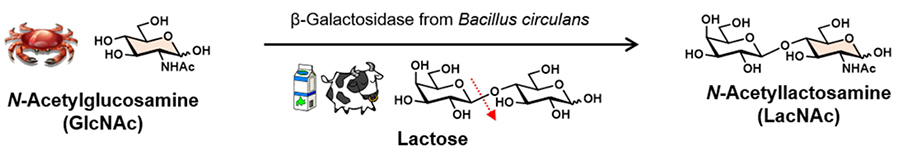
By focusing on the structures of oligosaccharides in glycoconjugates, we notice that several glycans contain GlcNAc as a constituent sugar. For example, at the base of N-linked glycans, the dimer of GlcNAc, N-acetylchitobiose (GlcNAcβ1,4GlcNAc), is bound to asparagine via an N-glycosidic bond, and N-acetyllactosamine (Galβ1,4GlcNAc; LacNAc) structures involved in molecular recognition are commonly present near the non-reducing end of N-linked glycans5. LacNAc is also a partial structure of biologically active oligosaccharides contained in human milk6. In other words, sugar materials prepared from chitin decomposition products, a marine biomass, can be used as materials for in vivo glycan research. Here, we introduce an in vitro enzymatic synthetic method of LacNAc using glycohydrolytic enzymes as an example. The glycosyl donor lactose (18 g, 50 mM) and the glycosyl acceptor GlcNAc (22 g, 99 mM) were dissolved in 50 mM sodium acetate buffer (pH 5.0), and then β-galactosidase (15 U) derived from Bacillus circulans was added. The reaction was carried out at 30°C for 24 hours, and the β-linkage-selective galactosyl transfer reaction to the 4-hydroxy group of GlcNAc proceeded, yielding 5.1 g of LacNAc, with a yield of more than 20% per glycosyl donor (Figure 2)7. Since almost no by-products are produced in this reaction, LacNAc can be easily isolated and purified. Furthermore, GlcNAc used as a sugar acceptor can be recovered and reused. This method, called the Usui method after its developer, is an innovative method for mass production of expensive in vivo oligosaccharides using inexpensive lactose and GlcNAc, a biomass carbohydrate7. There are other in vitro enzymatic synthesis methods for oligosaccharides that use glycosyltransferases and carbohydrate phosphorylases, so if you are interested, please refer to the references8,9.
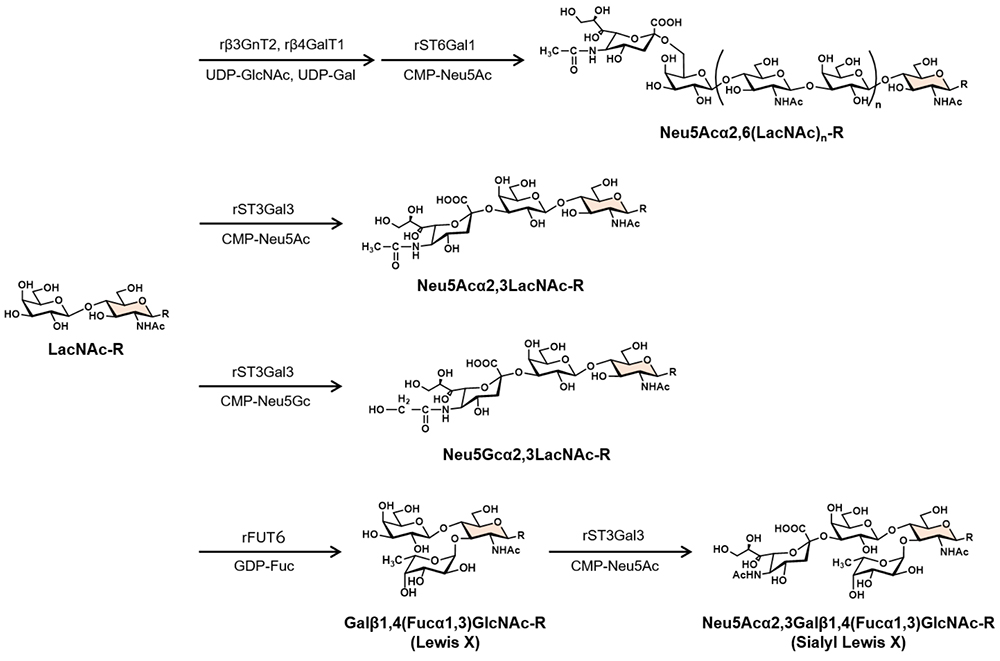
Furthermore, by combining LacNAc with various sugar nucleic acid donors and glycosyltransferases, it is possible to enzymatically synthesize various functional glycans involved in in vivo molecular recognition, such as influenza virus receptors Neu5Acα2,6(LacNAc)n (human type), Neu5Acα2,3LacNAc (avian type), and Neu5Gcα2,3LacNAc (horse type), as well as blood group glycan antigens Galβ1,4(Fucα1,3)GlcNAc (Lewis X antigen) and Neu5Acα2,3Galβ1,4(Fucα1,3)GlcNAc (sialyl Lewis X antigen), which are important for cell adhesion (Figure 3)10-12.
By regarding functional glycans, which can now be supplied in large quantities, as molecular recognition elements and introducing them into polypeptides, proteins, lipids, and other materials, it is possible to synthesize neo-glycoconjugates that mimic natural glycoconjugates13. The advantages of these neo-glycoconjugates are that they have a clear structure, can be prepared in required amounts, and can be designed and given material properties superior to those of natural materials. Such artificially created groups of biofunctional molecules, called neo-glycoconjugates, are being developed by many researchers on a daily basis and are being applied to biological science research. Here, we introduce the results of our research on the synthesis of artificial mucins and their application to adsorbents for proteins and pathogenic viruses. Mucins are a general term describing compounds in which glycans are multivalently attached to a core protein. It has been revealed that multivalently attached glycans induce a glycocluster effect and are involved in various biological interactions14.
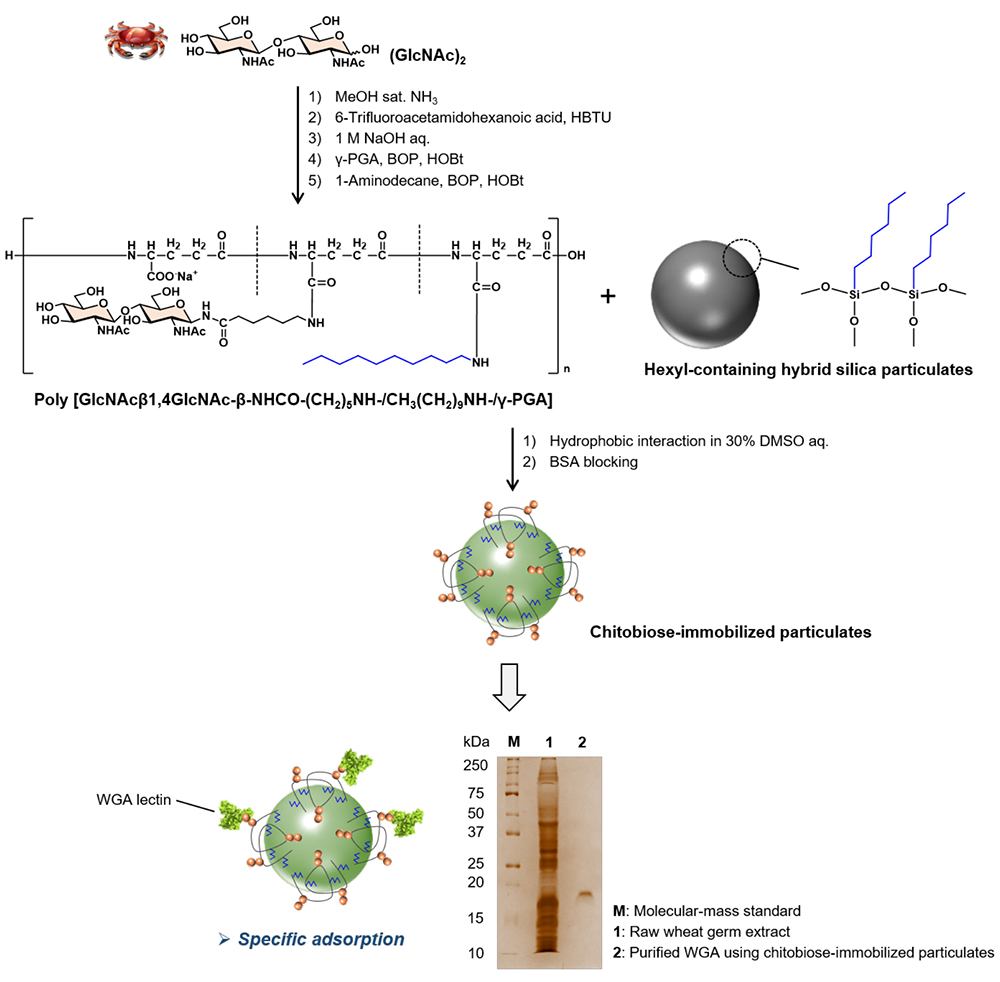
First, after preparing glycosylated (GlcNAc)2 from chitin decomposition products, we prepared a chitobiose-containing glycopolypeptide that mimics mucin in vivo by introducing about 20% of glycosides to the side chain carboxy group of γ-polyglutamic acid, a polypeptide produced by microbial fermentation. Furthermore, a chitobiose-containing glycopeptide with a hydrophobic group was synthesized by introducing an additional approximately 20% of decyl groups to the remaining side-chain carboxy groups in the chitobiose-containing glycopolypeptide. Subsequently, we demonstrated that the glycopolymer could be very easily coated onto the particle surface by hydrophobic interactions by simply shaking and mixing the separately prepared 1 μm diameter surface-hexylated silica particulates with the hydrophobic-group-introduced chitobiose-containing glycopolypeptides in a 30% dimethylsulfoxide (DMSO) aqueous solution for several hours (Figure 4)15. Furthermore, we demonstrated that the polyvalent glycans on the surface of the synthesized chitobiose-immobilized silica particulates exhibited glycan clusters as molecular recognition elements as designed, and functioned as an adsorbent with excellent binding affinity and specificity for the target protein. In an experiment using the synthetic particulates as affinity adsorbents, wheat germ lectin, a GlcNAc-binding lectin from raw wheat germ extract, was adsorbed in a structure-selective manner, and further, one-step purification of the target lectin was possible by removing the adsorbent from the particle surface (Figure 4)15.
Based on these results, we designed a virus adsorbent with glycan structures involved in adhesion to and infection of host cells by equine influenza virus particles and multivalently bind to the particle surface. Equine influenza infection is a term used to describe acute respiratory diseases caused by infection with the equine influenza virus. The virus is extremely contagious and equine influenza is the most alarming epidemic disease faced by people working in horse-related industries. Like human and avian influenza viruses, equine influenza viruses infect by the binding of hemagglutinin (a glycan-binding protein) to horse-specific N-glycolylneuraminic acid (Neu5Gc)-containing glycans on the surface of equine respiratory cells16. We immobilized Neu5Gcα2,3LacNAc, which was prepared and synthesized from chitin decomposition products, on the particle surface using the method described above to produce Neu5Gcα2,3LacNAc-immobilized silica particulates (Figure 5A)17. These particles selectively adsorbed the virus from nasal swabs obtained from horses infected with equine influenza virus (A/equine/Malaysia/M201/2015). Furthermore, by combining our developed equine influenza virus adsorption technique with real-time reverse transcriptase-polymerase chain reaction (rRT-PCR), we successfully were able to detect trace amounts of virus at the early stage of infection, which had been difficult to detect until now (Figure 5B)17.
In this article, we focus on chitin decomposition products industrially produced from chitin, and introduce biofunctional molecules with molecular recognition capabilities that have been designed and synthesized using these products as raw materials. As mentioned in the introduction, many different types of manufacturing processes utilize chitin, making it a truly fascinating research material that always entertains researchers. In this article, we have mainly introduced the development of molecular recognition materials that utilize chitin monosaccharides and disaccharides. However, our research group is also working on the total synthesis of simple bioactive substances using GlcNAc18, the development of enzyme inhibitors and substrates for enzyme activity measurement using (GlcNAc)419-22, and the development of an injectable gel using (GlcNAc)6, which we hope to introduce on another occasion.