
Miriam N. Ojima
Postdoctoral Researcher, Graduate School of Biostudies, Kyoto University, Japan
Bachelor of Science in Biology and Secondary Education, Florida State University
Master of Science in Biology, Georgia Institute of Technology
Ph.D. in Life Science, Kyoto University, JSPS Doctoral Fellow
Miriam N. Ojima studies the ecological role bifidobacteria play within the infant gut microbiome and hopes to apply her research to the development of efficient probiotic therapies.
An increasing body of work has shown that the gut microbiota plays an important role in human health and disease 1–5, and the microbial colonization of the infant gut that occurs postpartum is a critical period for gut microbiota development. Furthermore, the gut microbiota that forms during infancy can have long-term effects that last into adulthood5. As a result, therapeutic interventions aimed at regulating the gut microbiota, such as probiotics (microorganisms administered exogenously) and prebiotics (indigestible compounds that promote the growth of specific gut microbes), have become increasingly popular. For example, probiotic taxa such as bifidobacteria and prebiotics like human milk oligosaccharides (HMOs) are administered to encourage the development of a healthy gut microbiota in preterm infants6–8. Despite increasing use, studies report conflicting results and host response to microbiota-based therapies are highly variable. Based on recent in vitro and in vivo studies, I discuss how ecological processes like priority effects (i.e. the effect of species arrival order on community structure) and the presence of prebiotic HMOs can alter the structure of the infant gut microbiota and influence the effectiveness of such microbiota-based therapies.
The postpartum microbial colonization period that begins at birth is critical for gut microbiota development, and bifidobacteria are among the first colonizers of the infant gut. Bifidobacteria are Gram-positive and anaerobic bacteria that were first isolated from breastfed infant feces9, and they can occupy more than 70% of the gut microbiota in breastfed infants10–12. The presence of indigenous bifidobacteria in the infant gut has been linked to a variety of health effects, such as promoting immune system development13, preventing allergy and atopic dermatitis14, and reducing gut inflammation15,16. As a result, bifidobacteria are often administered as probiotics to preterm infants who are expected to have aberrant gut microbiota development17–19. Early life gut colonization by bifidobacteria is facilitated by their ability to utilize human milk oligosaccharides (HMOs) 20,21, which is a group of complex carbohydrates found in breastmilk with over 200 characterized structures22. Although HMOs are the third most abundant solid component in breastmilk, they do not provide direct nutritional value to the infant as they are resistant to pancreatic digestion23. Instead, they act as natural prebiotics that selectively promote the growth of certain gut microbes like bifidobacteria20,24,25. Bifidobacteria have evolved a variety of specialized strategies to utilize different HMO structures, and therefore there has also been an increased effort to add bifidogenic prebiotics like HMOs to infant formula.
Several clinical studies have tested the effect of administering bifidobacteria to infants. However, results are conflicting as some studies showed that it provided health benefits, while others have reported that it has no impact on improving health. One possible factor for this discrepancy is the timing of administration. As the infant gut is in the early stages of community assembly, niche availability for gut microbes is significantly affected by the timing of arrival. Ecological theory suggests that earlier arriving species can gain access to available niches first and therefore have an advantage in the community due to priority effects, in which the order and timing of microbial species arrival dictate the structure of the gut microbiota26,27, and clinical studies indicate that the first 24 hours of life is especially critical. For example, administration of Bifidobacterium breve BBG-001 to a group of preterm infants (median age at the first dose: 44 hours) did not significantly reduce the incidence of necrotizing enterocolitis (NEC) and colonization by bifidobacteria was not observed28. However, significantly increased gut colonization by bifidobacteria was observed when the first dose of B. breve was administered within a few hours of birth8,29,30. Health benefits are conferred by bifidobacteria when early administration allows for bifidobacteria to occupy available niches, thereby inhibiting colonization by taxa associated with disease and/or promoting the growth of other Bifidobacterium species31. In a different study, the combination of early life administration of Bifidobacterium longum subsp. infantis EVC001 and breastfeeding provided the optimum conditions for colonization by bifidobacteria32. Long-term colonization by bifidobacteria was made possible through priority effects, as well as the fitness advantage conferred by HMOs, which acted as prebiotics.
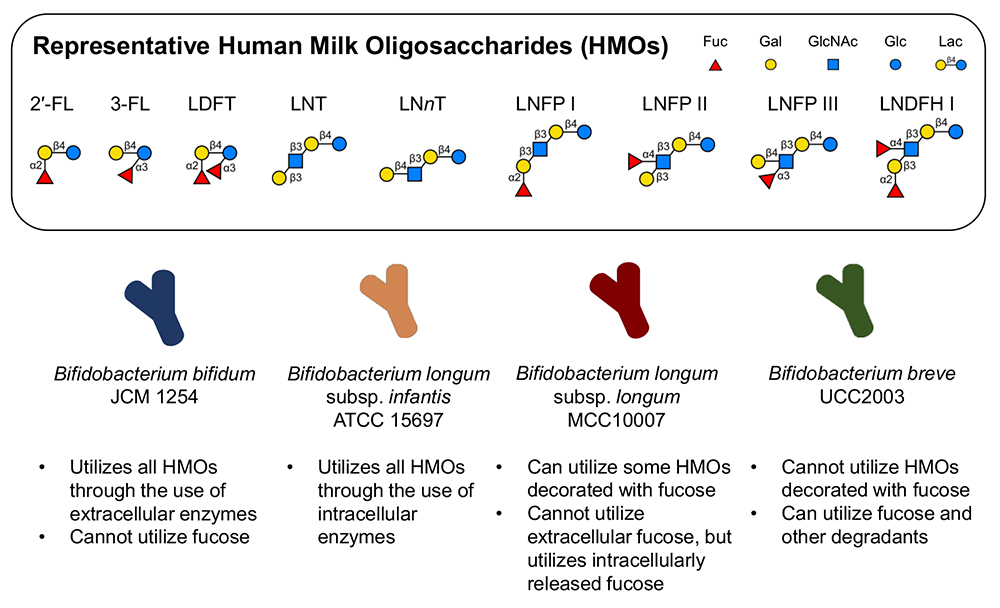
The role of priority effects in gut microbial communities has gained interest in recent years33,34, and the presence of prebiotic HMOs is expected to further facilitate colonization by bifidobacteria in the gut. However, the ability to utilize HMOs varies significantly by species and strain20,21. In addition to arrival order, relative fitness differences based on sugar utilization phenotypes, also determine community structure, as demonstrated in an experimental system using four strains of bifidobacteria (Bifidobacterium bifidum JCM 1254, Bifidobacterium breve UCC2003, Bifidobacterium longum subsp. infantis ATCC 15697T (B. infantis), and Bifidobacterium longum subsp. longum MCC10007 (B. longum)) cultured in medium containing HMOs purified from pooled breastmilk (Fig. 1) 35. These four species are often found in the infant gut and employ varying strategies for HMO utilization. B. bifidum JCM 1254 and B. infantis ATCC 15697T are known to assimilate the full range of HMOs through extracellular and intracellular glycoside hydrolases, respectively, and are therefore expected to be strong competitors. B. breve UCC2003 is expected to be a weak competitor as its HMO utilization ability is limited, although it can utilize HMO degradants like fucose (Fig. 1).
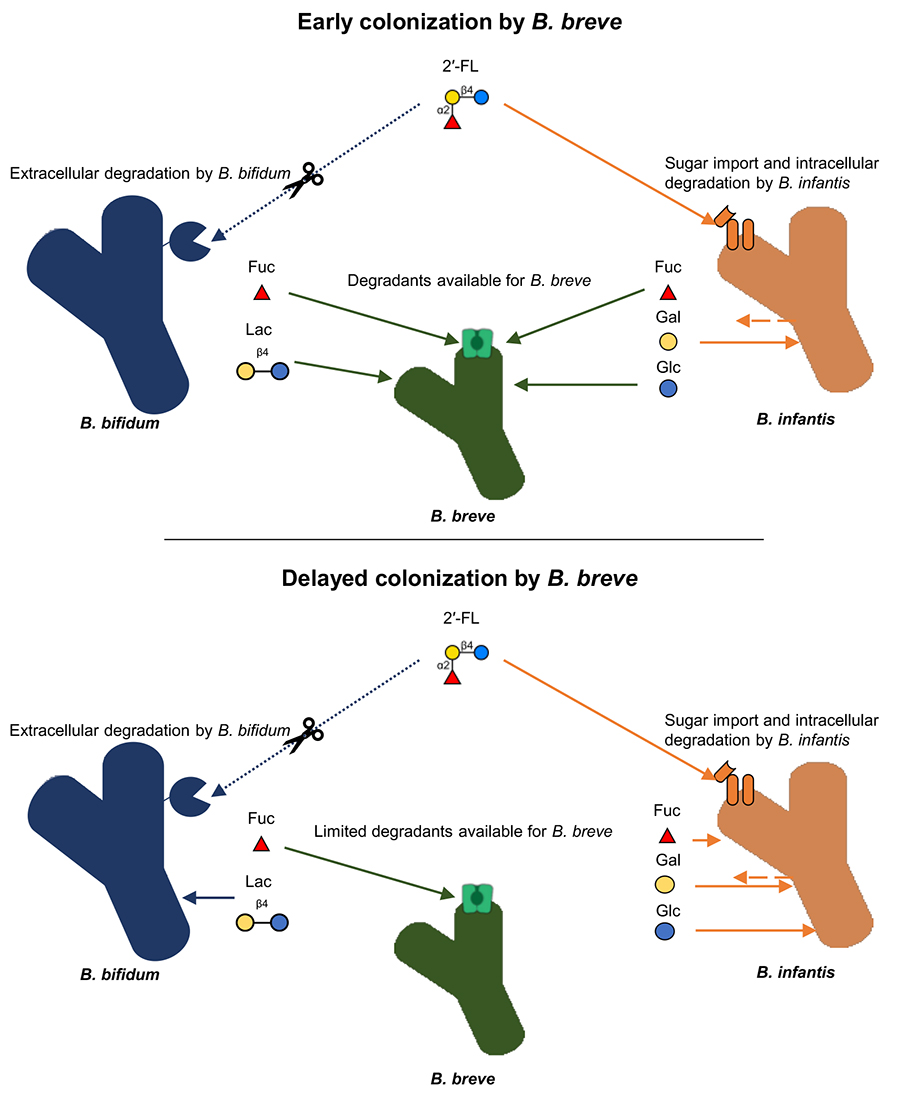
The effect of arrival order was apparent when strong competitors, B. bifidum and B. infantis, were introduced into the community first. B. bifidum and B. infantis dominated through inhibitory priority effects (i.e. depleting resources (HMOs) and making them unavailable for the later-arriving species). On the other hand, when B. breve was introduced into the community first, it outcompeted other species despite limited HMO-utilization ability. Rather, B. breve dominated due to its ability to utilize fucose, a monosaccharide component and degradant of HMOs provided by other species. Both B. bifidum and B. infantis provide fucose as degradants during HMO metabolization: B. bifidum degrades HMOs extracellularly to provide fucose and other HMO degradants36, while B. infantis imports HMOs whole and degrades them intracellularly, but temporarily excretes fucose and other monosaccharides37. Consequently, the early introduction into a community allows for B. breve to proliferate by utilizing HMO degradants like fucose as soon as it is made available by other species (Fig. 2). Patterns consistent with experimental data were seen in in vivo systems as well. Fecal metagenomic data from a cohort of European infant-mother pairs showed that when B. breve was present at birth, it was more likely to be dominant at 4 months of age38. These results highlight how the early-arriving species can affect the trajectory of community structure development and how the underlying mechanisms are influenced by species-specific competitive strategies and sugar consumption phenotypes.
As the gut microbiota formed during early life can have lifelong effects on health, there is an increased effort to implement microbiota-based therapies during infancy. Therefore, there is a need to understand the mechanisms by which probiotics and prebiotics provide health-promoting effects. Both clinical and experimental studies indicate the impact priority effects can have on the developmental trajectory of the gut microbiota. With bifidobacteria, the ability to utilize HMOs and strain-specific phenotypes, in addition to the timing of administration, are factors that should be considered to maximize their effectiveness as probiotics.
I would like to thank the following individuals for their constructive feedback on this paper: Dr. Takane Katayama, Dr. Avery Scherer, Dr. Xiulin Gao, and Dr. Grace Cagle.