
Tetsuro Ujihara
Technical Research Laboratories, Kyowa Hakko Bio Co., Ltd.
2006, Master of Philosophy, Graduate School of Arts and Sciences, University of Tokyo.
2006, Research Scientist, Kyowa Hakko Kogyo Co., Ltd.
2015, Doctor of Philosophy, Graduate School of Arts and Sciences, University of Tokyo.
2019-present, Senior Research Scientist, Kyowa Hakko Bio Co., Ltd.
I am interested in microbial production of valuable products including human milk oligosaccharides.
In particular, I am trying to construct useful strains and find processes for efficient large-scale cultivation.

Yanashima Kentaro
2007, Master of Science in pharmaceutical sciences, University of Tokyo.
2007, Research Scientist, Kyowa Hakko Kogyo Co., Ltd.
2017, Master of Business Administration, INSEAD
Present, Project leader for HMO development, Kyowa Hakko Bio Co., Ltd.
He has experience in new product development at Kyowa Hakko Bio after engaging in process development research at its research center until 2012. Since 2019, he has been managing the development of human oligosaccharides at Kyowa Hakko Bio and active in liaising the process development, engineering, quality assurance, regulatory affairs and marketing teams in order to launch three HMOs, 2’-fucosyllactose, 6’-sialyllactose, and 3’-sialyllactose from its own plant located in Thailand by 2022.
Human milk oligosaccharides (HMOs) is the general term for the complex sugar molecules (oligosaccharides) in human breast milk. HMOs are known to be important nutrients for infants because of their great abundance in human colostrum but only trace amount in bovine milk1-3 (Fig. 1). Therefore, efficient large-scale production of HMOs is desired to enhance the nutritional value of infant formula. However, HMO production was not easy. Limited numbers of HMOs can be obtained by using natural extraction methods4. Although there are several reports on the chemical synthesis of HMOs including 2'-FL, chemical synthesis of HMOs is still inefficient for industrial production purposes. HMOs consist of saccharide moieties with numerous hydroxy groups and should be attached in a stereospecific manner. In order to reduce the synthesis of unexpected byproducts, protection of the hydroxy groups is required, further complicating synthesis5. Enzymes which evolved over time can be used to easily produce complicated oligosaccharide chains with position-dependent activity in a stereospecific manner. So, nowadays, most HMOs are produced biologically.
Biological production of HMOs poses several challenges. The first is to discover the appropriate glycosyltransferase to produce the specific oligosaccharide structure. The second is to obtain enough substrate for the glycosyltransferase. Glycosyltransferase requires activated sugars in the form of nucleotide sugars. Nucleotide sugars such as UDP-Glc, UDP-Gal are expensive and unstable materials. The third challenge is to maintain high quality. To reduce impurities and unexpected byproducts, purification steps are also important. To overcome these challenges, many attempts have been made to date. We would like to use sialyllactose production as an example.
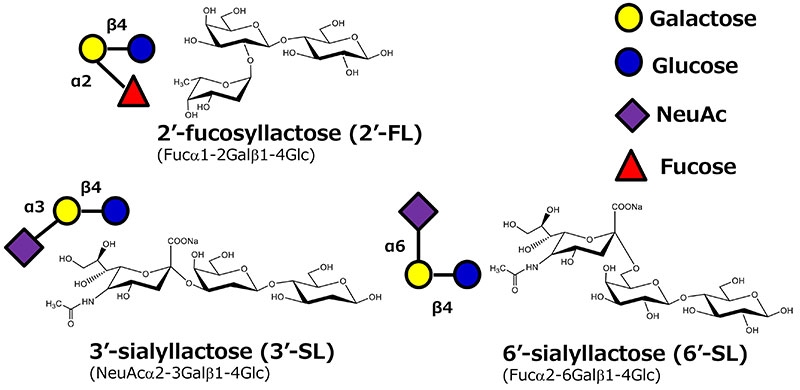
Sialyllactose consists of 6'-sialyllactose (6'-SL) and 3'-sialyllactose (3'-SL), both of which are major acidic HMOs in human breast milk. The acidity of sialyllactose is derived from the sialic acid (NeuAc) moiety. When sialic acid is linked to lactose by an alpha 2,3 glycosidic bond, it is called 3'-SL. And for 6'-SL, the glycosidic bond type is alpha 2,6. The glycosyltransferase for sialyllactose production is called sialyltransferase6 (Fig. 2). There are several sialyltransferases for 3'-SL and 6'-SL production. Sialyltransferases from the genus Photobacterium are used for 6'-SL production and sialyltransferases from the genus Neisseria, Pasteurella, or Campylobacter are used for 3'-SL production. The reason these bacteria possess sialyltransferases is still unclear. But one hypothesis is that exopolysaccharides with a similar structure to the human saccharide chain at cell surface of these bacteria are used to escape human immune system recognition7.
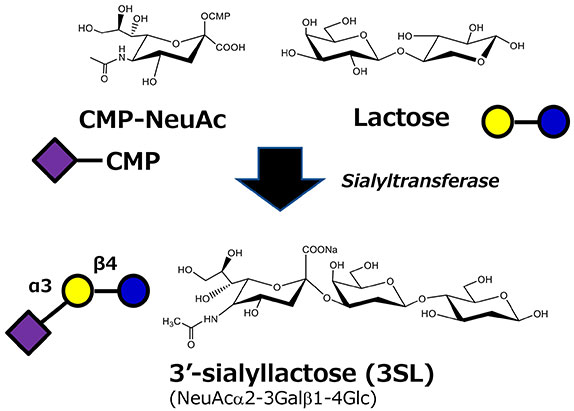
Sialyltransferases require CMP-NeuAc as their substrate (Fig. 2). Therefore, a lot of CMP-NeuAc is always needed for large-scale production of SLs. As CMP-NeuAc is a precious, unstable molecule, production of CMP-NeuAc has been a major barrier to SLs production.
CMP-NeuAc can be produced from NeuAc and CTP with CMP-NeuAc synthase. But NeuAc and CTP are each highly expensive materials.
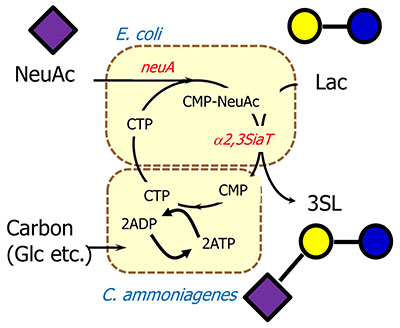
During SL production, CTP is converted to CMP-NeuAc and then released as a form of CMP. CMP can be regenerated to CTP by CMP kinase and nucleotide diphosphate kinase. As these two enzymes require ATP for phosphorylation of CMP and CDP, it could be said that ATP production/regeneration is the key factor. The first industrially successful ATP regeneration method was discovered by Kyowa Hakko Kogyo and the method used Corynebacterium, which has excellent ATP regeneration capabilities7 (Fig. 3). In this method, E. coli is used for expression of the appropriate enzymes such as glycosyltransferases, and Corynebacterium is used for ATP regeneration. These two bacteria are permeabilized by xylene or surfactants. With the aid of Corynebacterium cells, the enzymatic process which requires ATP is allowed to proceed. This method is used for industrial-scale production of CDP-choline or other materials7.
Other methods of ATP regeneration can be used such as ATP regeneration mediated by polyphosphate kinase and polyphosphate8. In this process, polyphosphate kinase is expressed in SL-producing recombinant E. coli. Although this process is simple and uses only E. coli, it requires relatively expensive polyphosphate. So, in terms of variable cost, the ATP regeneration system using Corynebacterium would be superior because it only uses inexpensive carbon sources such as glucose.
The next topic is the supply of sialic acid (N-acetylneuraminic acid, NeuAc). Sialic acid is found naturally in swallow's nests, but its content is not high. The first enzymatic process for NeuAc production was created as follows. The starting material was N-acetylglucosamine (GlcNAc), which is relatively inexpensive and readily available. Then GlcNAc was epimerized under alkaline conditions to produce N-acetylmannosamine (ManNAc). Finally, the ManNAc was converted to NeuAc with the reverse reaction catalyzed by N-acetylneuraminic acid lyase, which has the ability to combine pyruvic acid and N-acetylmannosamine9.
However, this reaction requires a large amount of pyruvic acid as a raw material and is not efficient enough. A more efficient method is to combine phosphoenolpyruvate with N-acetylmannosamine using sialic acid synthase. Since phosphoenolpyruvate itself is a high-energy compound, it must also be produced from inexpensive carbon sources such as glucose and glycerol in Corynebacterium or E. coli. Incidentally, N-acetylmannosamine can also be produced from glycerol and glucose, and it is possible to produce sialyllactose in a single strain (Fig. 4). This simple fermentation method is the most commonly used industrial process for SL production.
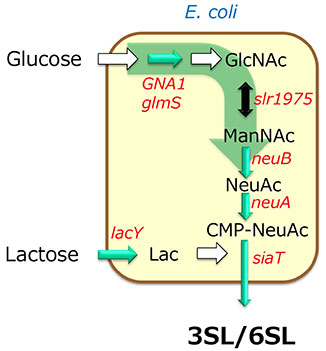
We have focused on SL production so far, but the basic problems are almost the same for other HMOs such as 2'-FL, 3-fucosyllactose (3-FL). GDP-fucose is used for the production of fucosylated oligosaccharides instead of CMP-sialic acid, UDP-GlcNAc is used for the transfer of N-acetylglucosamine residue, and UDP-Gal and UDP-Glc are used for the transfer of galactose and glucose. ATP is also required for the production of these sugar nucleotides, and the search for glycosyltransferases and an appropriate ATP regeneration system is key to these HMO production approaches (Fig. 4).
HMOs produced by fermentation or enzymatic reaction methods contain many impurities, including bacterial cells and residual substrates. Therefore, a purification process is essential for industrial production. Bacterial cells and other insoluble impurities can be separated by centrifugation or membrane separation. After the cell removal, supernatants are purified using ion exchange resins, and in some cases, desalted by electrodialysis. Finally, HMO powder is obtained by spray drying or crystallization.
Crystallization is achieved by adding organic solvents to lower the solubility of HMOs. During crystallization it is important to control the crystal form. Crystals of 2'-FL, 6'-SL, 3'-SL, etc. have been reported and are expected to be utilized in the production of high-purity products10,11 (Fig. 5). Unlike crystallization, spray drying does not improve purity, but it is attracting attention as a simpler and less expensive process. When spray drying is used, the purification process is important because the spray-dried solution must contain the target product at high purity. For the purification of acidic HMOs such as sialyllactose, ion exchange resins can be used to remove neutral and acidic impurities and chromatographic separation can be used to remove acidic impurities.
As we have described above, with the development of the biotechnological process, industrial-scale production of HMO has recently become feasible. 2’-FL and lacto-N-neotetraose (LNnT) have been launched by biotech startups in mid-2010s and infant formulas with 2’-FL and LNnT have been marketed since 2017. Additional HMOs such as 6’-SL, 3’-SL, and 3-FL have also become available quite recently and new infant formula containing those additional HMOs can be found in some countries.
HMOs are mainly used as additives in infant formula production. It is widely recognized that mother’s milk is the gold standard of baby formula. Thus, infant formula manufacturers are developing products that mimic human breast milk as much as possible. However, while there are more than 200 species of HMOs found in breast milk, only about seven HMOs (2’-FL, LNnT, 6’-SL, 3’-SL, 3-FL, LNT, difucosyllactose) have been commercialized so far globally12. However, the availability of HMOs depends on the regulatory situation in each country. For example, no HMO has been marketed in Japan while seven HMOs can be used in the US12. HMOs suppliers including Kyowa Hakko Bio are working on regulatory clearance of HMOs in several countries for marketing purposes. Nowadays, we see more and more countries that are opening their markets to products containing HMOs and more and more HMO-containing formulas that are being launched globally (Fig. 6).
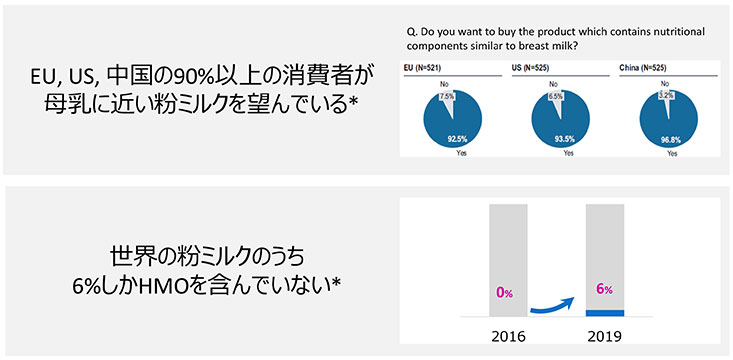
A consumer survey by Kyowa Hakko Bio found that more than 90% of the consumers in Europe, China, and the US want infant formula to be more like human breast milk (Fig. 7). There had been no HMO-containing formula before 2016, but, in 2019, 6% of infant formulas worldwide contained HMOs (Fig. 7).
In addition to working on HMOs to develop infant formula products, food companies are now starting to work on HMOs to develop new functional food products. For example, immunity, anti-infection, and brain function are the key areas of food industry interest. As the functional mechanisms through which microbiota exert their effects is one of the most interesting topics in HMO research, many companies are becoming interested in probiotic combinations, and HMOs as prebiotics.
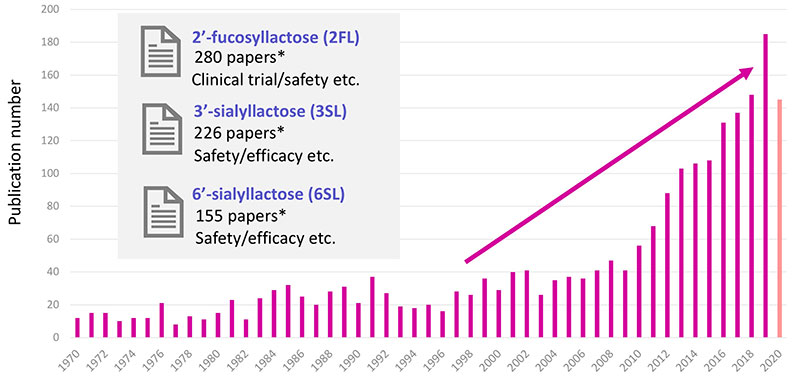
The number of articles has been increasing remarkably since 2010, which suggests that the supply of a large number of HMOs samples has enabled researchers to conduct more functional studies (Fig. 8). There are a lot of articles on 2’-FL already. We believe that it’s because the concentration of 2’-FL is high, and commercial products have become widely available. In parallel, the number of publications on other HMOs like 3’-SL and 6’-SL is increasing.
Several functionalities of HMOs have already been reported. Each HMO is reported to have unique effects. There are reports of anti-infection against Streptococci, Rotavirus, Norovirus, Influenza virus13,14 and immune-modulatory effects such as inflammatory cytokine suppression and food allergy relief15,16. For brain function, the increase of ganglioside, improvement of memory, suppression of anxious behavior, and gut-brain relationship are being investigated17-20. While known to support the growth of Bifidobacterium and Lactobacillus, HMOs are also expected to have inhibitory effects on pathogenic microorganisms in the gut21,22. Seeing the current advancements in probiotic research, we expect that probiotics suppliers will accelerate their R&D activities focusing on the combination of their own unique probiotic products and HMOs.
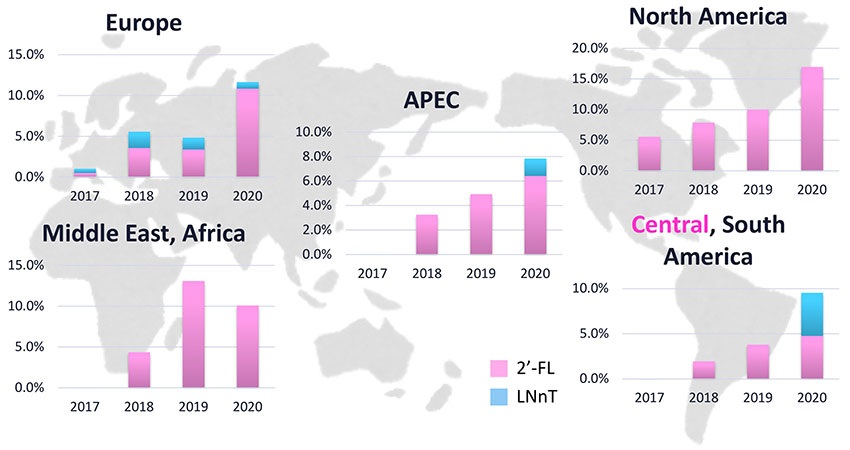
Consumption of 2’-FL is rapidly growing globally. HMO-containing infant formulas are sold now not only in the EU and the US, but also in parts of Asia including Vietnam, Singapore, and Hongkong. In 2021, Chinese regulators opened the door to HMO suppliers to register HMOs as food additives in China. This will enable infant formula manufacturers in China to add HMOs to their formulas. As China consumes around 50% of the global infant formula, HMO suppliers are now working to register their products in China23.
In 2010s, startups like Glycom and Jennewein Biotechnologie created the HMO market. In 2020, both of them were acquired by huge food ingredient companies. This indicates that the HMOs market is entering an expansion phase. Glycom, a Danish startup, was acquired by DSM, which is a giant company selling nutritional ingredients globally. DSM announced that they will invest in HMO businesses and make HMOs their business growth driver. On the other hand, Jennewein Biotechnologie, a German startup, was acquired by Christian Hansen, which is a leading global manufacturer of probiotics. Christian Hansen also announced in their press release that they will invest in HMO businesses and accelerate their R&D activity on probiotic-HMO products24. Additionally, in November 2020, Kyowa Hakko Bio issued a press release about its investment in its production site in Thailand, THAI KYOWA BIOTECHNOLOGIES, where it intends to establish its new production line and launch 2’-FL, 6’-SL, and 3’-SL in 4Q202225. This will be the first HMO production site in Asia with several HMO items. This manufacturing site is expected to offer a stable supply of HMOs to customers in Asian markets, which we expect to grow dramatically in the coming years.
Even now, less than 10% of infant formula products in the world contain HMOs. If HMOs can be manufactured more stably, more people will have access to HMO-containing formulas. If more species of HMOs become available commercially, people will have access to formulas that are much closer to human breast milk than ever before. In parallel, research on HMOs will be accelerated as the HMOs become more available to academia. HMOs are the ingredients human mothers are making for their precious babies. We expect to see more human clinical trials whose goal is to discover why HMOs are important in breast milk. We expect that HMOs will have health benefits in human adults as well as babies.