
Hiroshi Seo
2018 Served as the General Counsel of the Japanese Society for Chitin and Chitosan.
2013-2018 Served as the Secretary General of the Japanese Society for Chitin and Chitosan
2005-2012 Professor, Shukugawa Gakuin College
2003-2005 Special researcher at Post-graduate Science and Engineering Course, Tokyo Institute of Technology.
2002 Retired from the Research Institute of Fuji Spinning Co.,Ltd. (now Fujibo Holdings, Inc.) after having worked as a researcher in various research subjects
2000 PhD, Graduate School of Environmental Earth Science,Hokkaido University
1994-1995 Vice President of the Japanese Society for Chitin and Chitosan.
1989 Founder of “The Chitin and Chitosan Study Group” (now, the Japanese Society for Chitin and Chitosan)
1972 Graduated from Department of Polymer Science , Faculty of Science, Hokkaido University and entered Fuji Spinning Co.,Ltd.
The natural polysaccharides chitin and chitosan are mucopolysaccharides that are extracted mainly from crab shells and shrimp shells. Both chitin and chitosan have high affinity to the living body and have been extensively studied in various fields. They also have interactions with microorganisms and thus research extends to the agricultural and food realms all over the world. Chitin and chitosan are each found not only in solution form, but also in the form of fibers, porous beads, and sponges, depending on the usage. As the research on chitin and chitosan nanofibers is progressing with cutting-edge technology, the effects of size and specific surface area on expanding the possibilities of application are highly expected.
Since deterioration of the earth environment was first commented on, many years have passed with fruitless progress made in alleviation of this trend. During the1960s and thereafter, societies have accelerated not only mass production, but also mass consumption, aiming at high-level economic growth. As a result, progression of various environmental contaminations and further environmental destruction have occurred.
In crab fishing, when canning is conducted on the mothership over a distant voyage, crab shells that are considered useless are usually dumped into the ocean, resulting in sea contamination; and when crabs with shells are unloaded, they are processed in a crab-handling facility near the fishing port, followed by release of drainage, which becomes a cause of pollution in the bay. But chitin and chitosan are derived from crustacean shells.
Starting in the second half of the 1960s, “Effective utilization of unused natural resources” was actively promoted as a policy of the Japanese government. The promotion involved the commencement of chitin and chitosan research, centered at universities in Hokkaido and Tottori Prefecture, where crabs are unloaded. This developed as a global trend, and led to the First International Conference on Chitin and Chitosan (ICCC) in 1977 (Boston, USA). At the conference, many presenters addressed manufacturing methods as well as raw materials of chitin and chitosan, while several were on applications such as utilization of chitosan as an aggregating agent 1, and wound cure–promoting effects of chitin nonwoven fabric, gel, and sponge 2.
Five years later, in 1982, the 2nd ICCC was held in Sapporo, triggering progression of research and development at domestic universities and companies. Many Japanese researchers were inspired by “Functions and effective utilization of chitin and chitosan” 3 described by Hirano and began to investigate chitin and chitosan. Furthermore, the phrase “crab shells are a treasure mountain,” announced in mass media including TV and magazines, aroused a big boom in interest, continuing until nearly 2000.
Since the 2nd ICCC at Sapporo, the Conference has been held every 3 years at different locations. As shown in Table 1, the conference site has mostly been limited to Japan, the USA, and Europe. During the 4th conference, held in Trondheim, Norway, we planned to create domestic societies in the west as well as Japan. In 1989, we established the Japanese Society for Chitin and Chitosan, and held the First General Meeting and Memorial Lecture. Before then, we had held symposiums on chitin and chitosan and have continued the activity into the present. In the USA as well, the American Chitin and Chitosan Society (ACS) was created and the 5th ICCC was held at Princeton, NJ, in 1991; thereafter, however, such a conference has not been held in the USA.
In Asian countries, the 1st Asia-Pacific Chitin and Chitosan Symposium (APCCS) was held in Malaysia in 1994, and since has been held on a rotating basis every 2 years. Similarly in Europe, International Conference of the European Chitin Society (EUCHIS) have been held on a rotating basis every 2 years since the first meeting at Brest (France) in 1995. In Latin America, the organization La Sociedad Ibero-Americana de Quitina (SIAQ) was created, and has functioned well since its 12th I CCC was held at Fortaleza (Brazil).
At the 14th ICCC held in Osaka in 2018, a proposal regarding construction of an International Federation of Chitin and Chitosan Societies was made by the EUCHIS, which is currently under consideration.
| Conference | Host City | Host Nation | Year |
| 1st | Boston | U.S.A. | 1977 |
| 2nd | Sapporo | Japan | 1982 |
| 3rd | Senigallia | Italy | 1985 |
| 4th | Trondheim | Norway | 1988 |
| 5th | Princeton | U.S.A. | 1991 |
| 6th | Gdynia | Poland | 1994 |
| 7th | Lyon | France | 1997 |
| 8th | Yamaguchi | Japan | 2000 |
| 9th | Montreal | Canada | 2003 |
| 10th | Montpellier | France | 2006 |
| 11th | Taipei | Taiwan | 2009 |
| 12th | Fortaleza | Brazil | 2012 |
| 13th | Münster | Germany | 2015 |
| 14th | Osaka | Japan | 2018 |
As for the number of participants in each past ICCC, Japanese participants have always followed the top, after the people of the host nation. Japanese participants give many presentations, which are mostly technologically advanced. Their studies have been quantitatively and qualitatively so excellent that they have been highly praised as playing a role model for other countries.
At the 14th ICCC and 12th Asia-Pacific Chitin and Chitosan Symposium, both held in Osaka in August 2018, there were a total of 230 oral and poster presentations. Among them, Japanese presentations accounted for over 38%. A total of more than 300 participants gathered from 31 nations worldwide. The ratio of Japanese participants to all ones was 42.4%. This conference provided us several benefits: of them, the first is participation and presentation by many young students (probably owing to being the host nation); and the second is therefore a large expectation toward the promotion of future research.
Chitin is present in the shells of crustaceans and insects. When chitin is hydrolyzed with alkali, chitosan is obtained. Chitin is described as poly-N-acetyl glucosamine, and chitosan as poly-glucosamine; however, neither exists as a homo-polymer in nature, but nearly 10% of each exists in a deacetylated form.4
Chitin possesses glucosamine residues. Accordingly, chitosan produced by deacetylation after hydrolysis of chitin can be called deacetylated chitin (DAC), and the structure of the obtained chitosan can be identified by the degree of deacetylation, if mentioned. On the other hand, deacetylated chitin had previously been called “chitin-chitosan,” but this name is an obvious mistake and has not been used.
The author and others have tried experiments on methods of preparing chitin from shells of crabs, shells of shrimp, and squid pens. Chitin derived from squid gladius has β-type crystal structure (called β-chitin) and differs from α-type chitin (α-chitin) representatively derived from crab shells. In β-chitin, glucose chains are parallel and hydrogen bonds are loose and advantageously manageable. However, as raw materials for production of chitin and chitosan, crabs are considered most preferable, because their size enables the highest yield. Among various kinds of crab, the red snow crab (Japanese queen crab), which has a thin shell and contains less calcium, is mainly used.
Here, a brief description regarding the preparation of chitin from the crab shell material is provided. In general, Hackman’s method is well known.5 That is, dry crab shells are immersed in 2N hydrochloric acid for 5 hours, then washed with water, dried, and smashed, and thereafter, subject to decalcification with hydrochloric acid under stirring for 48 hours. Next, the mixture is heated in 1 N sodium hydroxide at 100°C for 12 hours. This procedure is repeated 4 times to remove proteins and the residue is dried to obtain crude chitin. Subsequently, the crude chitin is immersed in 0.5% potassium permanganate solution for 1 hour, then washed with water and stirred in 1% oxalic acid solution at 60°C for 30 – 40 minutes to obtain pure white chitin.
To obtain chitosan from chitin, deacetylation reaction is conducted. Namely, chitin is allowed to react with 48% sodium hydroxide solution at 120°C for 30 minutes, and finally pure chitosan is yielded. Chitosan obtained via this route is easy soluble in organic acids such as acetic acid, lactic acid, and succinic acid.
The natural polysaccharide chitin possesses excellent affinity with the living body. Chitin is degraded by the bactericidal enzyme lysozyme, much contained in our tears and saliva. Utilizing this degradation in the living body, development of chitin-based surgical sutures that do not require removal was investigated. However, so far, ideal sutures composed of chitin fibers have not been realized, because several problems have not yet been resolved, including strength under humidity and residual solvent for chitin fibrosis. In addition, it was reported that chitin does not possess antigenicity. On the other hand, fabrics and sponges composed of nonwoven chitin were reported to be used as wound-covering agents and wound-filling agents, respectively, in cases of veterinary clinical application.6 Beschitin® (Nipro Pharma), a commercially available product that was approved as a specified care material among pharmaceuticals and medical devices or wound-covering agent for burns and pressure sores, is also on the market.7
The chitosan molecule possesses both amino groups and hydroxyl groups, and therefore it is very reactive and is easily subject to chemical modification. Bridging can be achieved with 2-functional reagents as well as acylation, etherification, and sulfonation.
Since chitosan has amino groups, not only its affinity to proteins, but also metal adsorption capacity is high. In addition, both its antibacterial and antifungal effects are high. Given these properties, development of expanded applications can be strongly expected.
As mentioned above, chitosan is easily soluble in organic acids such as acetic acid and lactic acid, and thus it can be secondarily molded into various forms. The author and others have been actively involved in expansion of applications, using chitosan fibers, porous chitosan beads, and fine chitosan powder. These are sequentially described below.
To create chitosan fibers, the following three methods are used: 1) copper-ammonia coagulation with CuSO4-NH4OH; 2) alkali neutralization with NaOH-EtOH; 3) copper-sulfuric acid coagulation with CuSO4-H2SO4.8
As the raw solution for spinning, 5% dichloride acetate aqueous solution of chitosan is used. On the chitosan fibers obtained, the adsorption amount of γ-globulin protein is measured. The highest adsorption amount is observed using method 1, above (Cu-NH4 coagulation). This finding indicates that the amino groups of chitosan turn outside under the presence of copper sulfate (CuSO4) in the coagulation bath.
Chitosan is dissolved in acetic acid to make dope, which is dropped into a coagulation bath intermittently through a nozzle to produce spheres. At this stage, adjustment of coagulation speed in the alkali coagulation bath (using mainly sodium hydrate) slows the speed of both desolvation and coagulation, finally leading to production of a continuous porous material. For example, chitosan (deacetylation, 90%; mean molecular amount, 80,000) is dissolved in 4% acetic acid aqueous solution to make 5% conc. chitosan dope. The dope is dropped into a NaOH/EtOH coagulation bath intermittently through a nozzle (diameter, 0.15 mm) to produce beads. Bridging with use of a bifunctional reagent then yields acid-insoluble beads.9 These beads are porous with a honeycomb structure, as shown in Figure 1, and have been produced with a specific surface area over 200 m2/g.
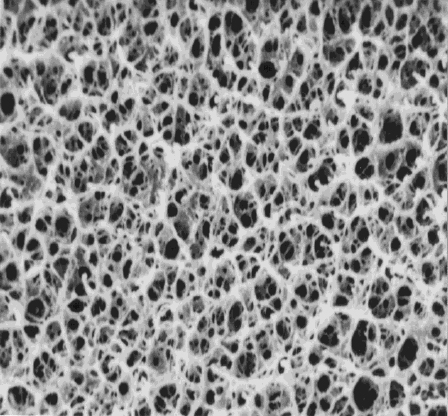
The author and a group used chitosan beads (diameter, 1 mm) to examine the adsorption of heavy metals in mine water, picked up in Shiribeshi district, Hokkaido. As shown in Table 2, the results demonstrate that chitosan beads highly adsorb zinc and cadmium. In addition, a comparison of bridged chitosan beads with non-bridged chitosan beads reveals that the former exerts a higher adsorption capacity than the latter, as a whole. This is because bridging increases specific surface area.9 In addition, it is considered that, in non-bridged chitosan beads, internal dissolution may be induced by the acidic aqueous solution containing metals, damaging the honeycomb structure.
| pH | Unit | Zn | Cd | Fe | Mn | ||
| Mine water | 3.45 | a) | mg/L | 184 | 0.28 | 301 | 22.5 |
| Chitosan beads | 6.32 | b)supernatant | mg/L | 83.8 | 0.012 | 120.4 | 19.4 |
| ratio (b/a) | % | 54.5 | 95.7 | 60.0 | 13.8 | ||
| Chitosan beads crosslinked | 6.48 | C)supernatant | mg/L | 18.6 | 0.0025 | 99.5 | 17.1 |
| ratio (c/a) | % | 89.9 | 99.1 | 66.9 | 24.0 |
An experiment was carried out in which chitosan beads were incorporated into a bioreactor as a vector for immobilized enzymes.
The Food Industry Bioreactor System Technology Research Association, subsidized by the Ministry of Agriculture, Forestry and Fisheries, started a 5-year project with a subject of “development of the bioreactor system in the food industry,” in fiscal year 1984. This project included a total of 20 subjects in carbohydrate-, protein-, and lipid-related research in coordination among industry, government, and universities. Chitosan beads were evaluated in 12 subjects10 of which representative findings are described below.
(1) Manufacture of starch sugar
The process advances as follows: liquefaction → glycosylation → isomerization.
To conduct these sequentially, a bioreactor system was developed. In the glycosylation process, bench-scale plant operation using fixed glucoamylase led to a conclusion that continuous glycosylation for 1,000 hours by 4 half-life is feasible.
(2) Manufacture of aged white soy sauce
The manufacturing process begins with enzymatic digestion of crude soybeans, followed by seasoning in the route from glutamine to glutamic acid, and finally flavoring with fixed soy sauce yeast. In the second process, a 4-liter glutaminase fixing reactor was used to produce 24 liters/day steadily over 2 months.
In the 1980s, the market was flooded with antibacterial and/or deodorant processed products and consumers were enthusiastic about taking antibacterial and deodorant measures against all things that can be touched by hand, including the receiver of public telephones and hand grips in trains and subways. As antibacterial agents, agricultural bactericides and metallic chemicals were commonly used.
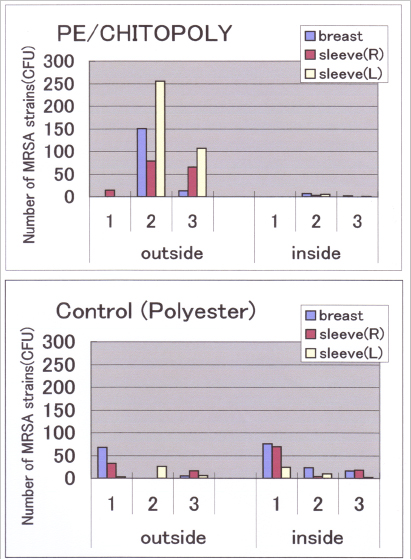
The author and a group considered that chitosan, having free amino groups, might exert antibacterial effects via ionic action, and conducted antibacterial/antifungal tests using chitosan in various microbiological organisms. Results are summarized in Table 3.11 For this purpose, chitosan was finely ground to particles less than 5 µm diameter, and then kneaded into polynosic rayon to prepare antibacterial deodorant fibers. This new rayon fabric was very effective against MRSA (Methicillin-resistant Staphylococcus aureus), well known as an in-hospital infectious pathogen. Furthermore, an isolation gown was prepared from this rayon and tested for its usefulness in a clinical study.12 As shown in Figure 2, the inside of the gown, consisting of nonwoven chitosan/polynosic fabric (CHITOPOLY) and polyethylene film ruminate, was almost free from MRSA, even though the outside of the gown was contaminated with MRSA.
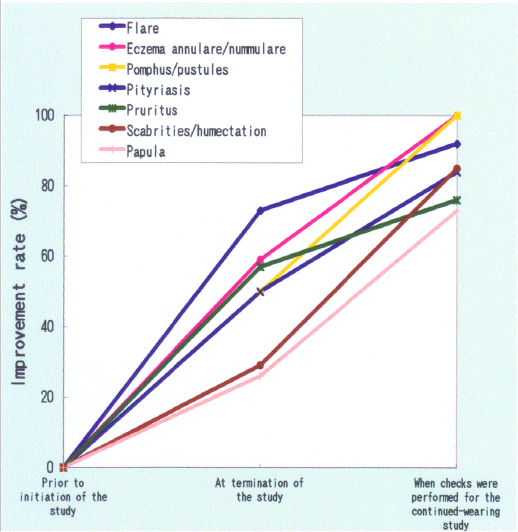
Separately, kneading the edible polymer chitosan as an antibacterial element into the chemical constituent cellulose polynosic rayon led to supply of very safe and highly moisturizing soft-touch fiber products, including undergarments, which can be used even by infants and children with atopic dermatitis.13 A follow-up investigation conducted six months after the end of a clinical trial on undergarment-wearing revealed that improvements of symptoms of atopic dermatitis were highly rated, as shown in Figure 3.14
| Bacteria | MIC value (ppm) |
| Agrobacterium tumefaciens | 100 |
| Bacillus cereus | 1000 |
| Corinebacterium michiganence | 10 |
| Erwinia sp. | 500 |
| Erwinia carotovora subsp. | 200 |
| Escherchia coli | 20 |
| Klebsiella pneumoniae | 700 |
| Micrococcus luteus | 20 |
| Pseudomonnas fluorescens | 500 |
| Staphylococcus aureus | 20 |
| Xanthomonas vampestris | 500 |
| Fungi | MIC value (ppm) |
| Botrytis cinerea | 10 |
| Fusarium oxysporum | 100 |
| Drevgslera sorokiniana | 10 |
| Micronectriella nivalis | 10 |
| Piricularia oryzae | 5000 |
| Rhizoctonia solani | 1000 |
| Trichophyton equinum | 2500 |
Nanofibers compared with the conventional micron-unit fibers are largely characterized by super-specific surface area effects, nano-size effects, and ultramolecular arrangement effects. The platform technology has been expected to be helpful in areas of energy, information, and correspondence, as well as life science, medical care, and biotechnology.15
The author and others have been successful in preparation of not only chitosan nanofibers, but also pectin nanofibers.16
A new book published as edited by the Japanese Society for Chitin and Chitosan shows that both chitin nanofibers and chitosan nanofibers have been produced according to various methods, including electrospinning technique, cathode electron weaving, mechano-chemical crushing, and water jet cutting/crushing.17 Furthermore, the feasibility of advancement has been proposed in the field of technical materials, including air filters and masks, the area of functional food to improve the intestinal environment, in cosmetics aiming at skin activation, and in the medical care field connected with wound healing and drug-delivery systems.18
Regarding chitin and chitosan, cases of use in clinical applications such as wound-covering, hemostatic agents, and animal care drugs have been accumulating.
In the future, applications in the medical field will progress on the basis of absence of antigenicity. Anraku, who engaged in experiments using obese spontaneous hypertensive rats, found that the antioxidative effect of low-molecular weight chitosan was associated with oxidative stress inhibition in blood. He also learned based on chronic renal insufficiency model rats that chitosan adsorbed uremic materials, and he then proposed a chitosan effect on metabolic syndrome and a suppressive effect on renal insufficiency progress.19
Moreover, Hayashi and others in the dental field are engaged in regenerative care for dentin and gene therapy research; 20 their results are very promising.