
Dr. Masellis received her B.Sc. degree from the University of British Columbia in Vancouver, British Columbia and her Ph.D. degree from the University of Alberta in Edmonton, Alberta. Her Ph.D. work focused on the role of the tetraspan molecule CD9 and integrin adhesion molecules in B cell adhesion. She also studied B cell interaction with bone marrow stromal cells, which directed her interest to postdoctoral studies in adhesion and migration of bone marrow derived plasma cells in multiple myeloma. She joined the Virginia Piper Cancer Institute at Abbott Northwestern Hospital in Minneapolis in 1998 and is an adjunct Assistant Professor in the Department of Medicine at the University of Minnesota. Her current research focus is tumor cell interactions with bone and includes hyaluronan-cell interactions in mesenchymal cell differentiation and disease.
Tumorigenesis is a multistage process, having an absolute requirement on coordinated events intrinsic as well as external to the cancer cell. It is well recognized that the tumor microenvironment plays a significant role in tumor progression. In particular, soluble as well as extracellular factors, including cytokines and growth factors, extracellular matrix molecules and cellular ligands, all influence the biological behavior of the tumor. Hyaluronan, a major constituent of the extracellular matrix, is a large negatively charged glycosaminoglycan composed of repeating units of N-acetylglucosamine and glucuronic acid. Hyaluronan has important roles in matrix assembly, cell proliferation, cell migration and embryonic/tissue development. Recent studies have provided evidence for hyaluronan and hyaluronan-cell interactions affecting not only cell migration and proliferation, but also the invasive potential of cells and malignant transformation. The biological functions of hyaluronan may be in part attributed to its physical properties (being a large negatively charged macromolecule), its associations with other extracellular matrix molecules (including aggrecan, versican, neurocan, brevican, TSG-6, link proteins) and involvement in signal transduction through interaction with cell surface receptors (CD44, Receptor for Hyaluronan Mediated Motility, Lyve-1).
The bone marrow microenvironment is a multi-functional network of cells and extracellular matrix that maintains hematopoietic cell proliferation, differentiation and survival (Fig. 1) 1. Among the important cell lineages from the bone marrow are those derived from the mesenchymal progenitor cell, including chondrocytes, adipocytes, fibroblasts and osteocytes 2. These cells differentiate along the various lineages in response to external microenvironment signals and internal lineage commitment signals. However these external and internal signals are not well understood. It is likely that many factors, including growth factors, cytokines and cellular as well as extracellular matrix, impact growth, differentiation and function of the bone marrow mesenchymal cells.
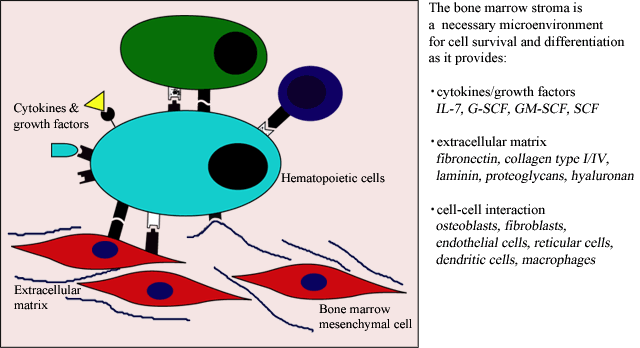
Fig. 1 The Bone Marrow in Hematopoiesis
The bone marrow extracellular matrix consists primarily of fibronectin, collagen types I and IV, laminin and the glycosaminoglycans heparan sulfate, chondroitin sulfate and hyaluronan. Hyaluronan, a major glycosaminoglycan of this matrix functions in multiple roles to influence bone marrow hematopoiesis and cellular differentiation. The influence of CD44:hyaluronan interaction in hematopoiesis has been demonstrated by the use of anti-CD44 mAbs or treatment with hyaluronidase; both treatments significantly reduce lymphopoiesis and myelopoiesis in vitro 3, 4.
Our laboratory is interested in how the expression of hyaluronan and hyaluronan synthase (HAS) enzymes in bone marrow mesenchymal cells, as well as how hyaluronan-mediated cellular interactions, might impact on the localization and survival of multiple myeloma plasma cells and other bone seeking tumors. Over the past several years, we have utilized an in vitro model system of bone marrow stromal cultures derived from both healthy donors and patients with multiple myeloma to define a role for hyaluronan in malignancies with bone involvement.
Hyaluronan and hyaluronan synthases appear to have a significant biological role in tumor progression, and, in certain malignancies, hyaluronan serves as a clinical prognostic indicator of disease progression. There is increasing evidence that hyaluronan and hyaluronan synthase genes are intricately involved in tumorigenesis, and in the process of angiogenesis 5-7. In particular, hyaluronan production is up-regulated in a variety of malignant tissues compared to normal cells, and increased hyaluronan levels as a consequence of HAS gene expression have been shown to correlate with increased tumor cell invasion 8-14. For example, over-expression of Has2 in fibrosarcoma cells or of Has3 in prostate carcinoma cells results in growth of larger tumors, while breast cancer cells transfected with Has1 have increased metastatic potential compared to parental cells. While the majority of studies have utilized solid tumor models, there is evidence that hyaluronan is relevant in hematological malignancies as well. Most recently in multiple myeloma, hyaluronan levels in serum are shown to correlate with poor disease outcome 15.
Multiple myeloma is an incurable plasma cell malignancy that accounts for 13% of lymphoid malignancies in the Caucasian population 16. This disease is characterized by the presence of monoclonal bone marrow plasma cells within the bone marrow (hereafter referred to as myeloma plasma cells), monoclonal protein, hypercalcemia, lytic bony lesions and anemia. An illustration of multiple myeloma development during B-cell maturity is shown in Figure 2. Treatment for myeloma is not curative, and the median survival is 3-4 years.
The bone marrow microenvironment likely plays a profound role in myeloma disease progression. The fact that myeloma plasma cells preferentially accumulate in the bone marrow implies that the bone marrow mesenchymal cells within this compartment best support the growth and maintenance of the myeloma clone. Co-culture systems have shown that physical cell-cell contact, soluble and matrix-associated growth factors/interleukins, and cell-matrix interactions affect the growth and survival of the myeloma bone marrow plasma cell and its response to chemotherapeutic agents (refer to Fig. 1). In addition to myeloma bone marrow plasma cells, there is speculation that a CD19+ B cell (hereafter referred to as myeloma B cell) exists within the peripheral blood of myeloma patients that may comprise a component of the malignant clone with the ability to intravasate from the peripheral blood into the bone marrow.
Others and we have shown that bone marrow mesenchymal cells derived from myeloma patients have altered adhesion receptor expression, cytokine expression and extracellular matrix expression. Despite the increasing acceptance of the influential role imparted by the bone marrow microenvironment on myeloma cells, few investigations have focused on the characterization of the bone marrow stroma, and the identification of any specific anomalies within this compartment, that may contribute to the pathophysiology of this disease.
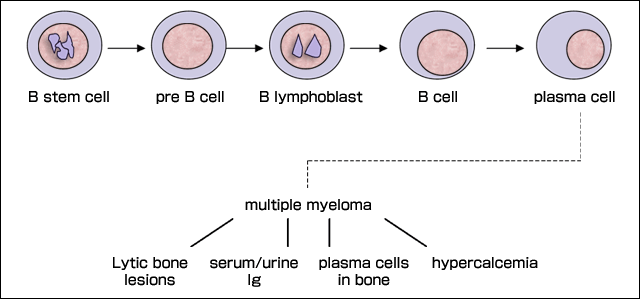
Fig. 2 B Cell Development and Multiple Myeloma
The overall metabolism of hyaluronan is determined by the enzymatic activities of hyaluronan synthases and hyaluronidases. Three hyaluronan synthase genes have been described, each encoding a plasma membrane protein responsible for hyaluronan synthesis 17. Although expression of any one hyaluronan synthase gene is sufficient for hyaluronan synthesis, the contribution of the three HAS gene products to hyaluronan production and expression in most systems is not yet clear. It has been suggested that the hyaluronan synthases possess unique enzymatic properties resulting in different kinetics of hyaluronan synthesis. For example, it is suggested that Has3 is responsible for the synthesis of low-molecular mass hyaluronan (< 2 x 105) while Has1 and Has2 are responsible for the synthesis of larger molecular weight hyaluronan (>2 x 106). Likewise, potential differences in enzymatic function of the various hyaluronan synthases may be very important in vivo and possibly relate to the various functions ascribed to hyaluronan.
As a first step towards discerning the role of hyaluronan as an integral component of the bone marrow extracellular matrix in multiple myeloma patients, the hyaluronan synthase gene expression and hyaluronan production in bone marrow mesenchymal cells derived from healthy donors and from multiple myeloma patients were characterized. The expression of hyaluronan synthase genes in healthy and myeloma bone marrow mesenchymal cells was determined by quantitative competitive Rt-PCR. Utilizing this method, it has been shown that bone marrow mesenchymal cells derived from myeloma patients express predominantly Has1. In contrast, bone marrow mesenchymal cells derived from healthy donors predominantly express Has2 (Fig. 3).
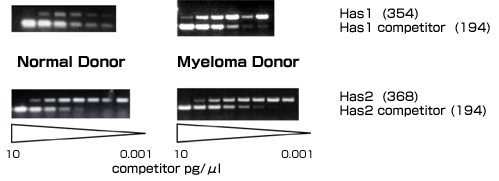
Fig. 3 cRt-PCR for Has1 and Has2 in Myeloma and Healthy Donor Bone Marrow Mesenchymal Cells
Total RNA was extracted from bone marrow mesenchymal cell cultures at no greater than 80% confluency. cRt-PCR was performed as indicated in reference 18. The cRt-PCR was performed with 1 µg/ml total RNA and recombinant Has DNA used as the internal competitor (constructed as described in ref. 18). The concentration of the Has competitor ranged from 10 pg/µL to 0.000 0001 pg/µL (a range of 0.001-10 pg/µl is shown).
The relevance of the various HAS isoform expression to overall hyaluronan synthesis and matrix assembly is not yet well characterized. Given that myeloma bone marrow mesenchymal cells express Has1 while the healthy donor counterparts express Has2, we could surmise that Has1 will impart a novel hyaluronan phenotype to myeloma cells. The consequence of Has1 expression may ultimately impact myeloma plasma cell motility, interactions between myeloma plasma cells and bone marrow mesenchymal cells, as well as impact on normal bone marrow mesenchymal cell function. However, it is yet to be determined whether signals initiated by cell-cell contact or soluble cytokines expressed by the plasma cells is involved in the regulation of HAS gene expression. For example, co-culture of myeloma bone marrow mesenchymal progenitor cells with the plasma cell line ARH77 reduces the Has1 mRNA expression by 80% (+/- 5%) in bone marrow mesenchymal cells while Has2 mRNA is unaltered. Still, given that co-culture of bone marrow mesenchymal cells with myeloma plasma cells results in cytokine up-regulation, it would be intriguing to speculate a potential autocrine feedback mechanism involved in the regulation of HAS genes in myeloma mesenchymal cells (Fig. 4).
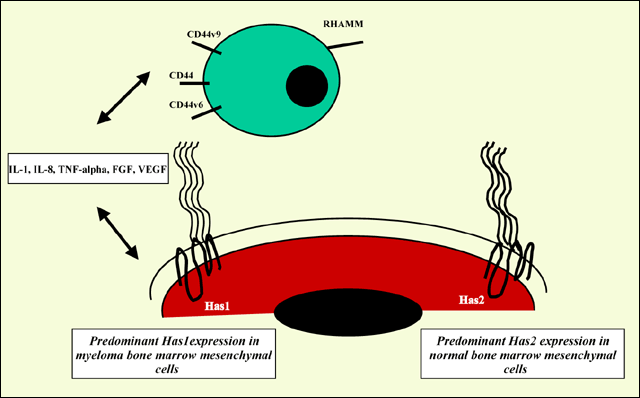
Fig. 4 A Schematic of Hyaluronan Synthase, Hyaluronan Receptors and Hyaluronan-Responsive Cytokines in the Myeloma Bone Marrow Microenvironment
The expression of HAS isoforms correlates with hyaluronan synthesis. We have shown that compared to healthy donor bone marrow mesenchymal cells, myeloma bone marrow mesenchymal cells secrete 9.6 fold more hyaluronan (Table 1), whereas chondroitin sulfate, a major glycosaminoglycan constituent of the matrix, is the same in the two groups 18. The hyaluronan associated with the cellular matrix is almost identical in myeloma bone marrow mesenchymal cells compared to healthy donor cells indicating that the excess hyaluronan is secreted into the culture medium (Table 1).
Table. 1 Relative Amounts of Hyaluronan in Medium and Cell Layers of Bone Marrow Mesenchymal Cells
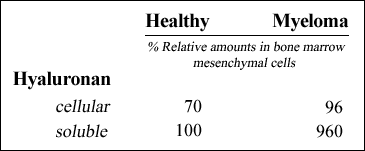
Fluorophore-assisted carbohydrate electrophoretic (FACE) analysis was used to measure the relative amounts of hyaluronan in cultures derived from either healthy donors or myeloma patients. Medium and cell layer fractions of 48-hour cultured cells were subjected to proteinase K digestion, ethanol precipitation and digestion with hyaluronidase SD, chondroitinase ABC and glucoamylase. To visualize carbohydrates, sample were subjected to fluorescent-derivatization with 2-aminoacridone and electrophoresed using MONOP composition gels. Data were obtained in collaboration with Dr. A. Calabro (Department of Biomedical Engineering, Cleveland Clinic Foundation). See reference 18 for further materials and methods.
It would be particularly interesting to determine how expression of the various HAS enzymes in mesenchymal cells might differentially regulate hyaluronan expression and consequently matrix organization, as well as hyaluronan-mediated interactions with myeloma plasma cells within the bone marrow of myeloma patients. Similarly, the role of hyaluronan-binding proteins in hyaluronan matrix assembly is as yet an unexplored area in the biology of bone marrow mesenchyme but may be as important as HAS isoform expression and hyaluronan synthesis.
Recently, both abnormally low or high hyaluronan concentrations in serum have been shown to correlate with disease progression in multiple myeloma 15. Although there is yet no indication that abnormal hyaluronan levels induce shorter overall survival of patients, it may be that hyaluronan serum level may be indicative of the pathological course of this disease. In that regard, hyaluronan levels in the serum may be prognostic for a select group of myeloma patients with adverse outcome. Although the clinical usefulness of hyaluronan in myeloma is not yet certain, it is apparent from a multitude of studies (cited below) that hyaluronan and hyaluronan receptors are involved in a variety of biological aspects in this disease.
Hyaluronan Receptors and Adhesion in Myeloma: Myeloma cells express multiple adhesion receptors, including the hyaluronan receptors CD44 and RHAMM (Receptor for Hyaluronan Mediated Motility) 19. With regards to CD44, myeloma plasma cells exclusively express CD44v3, CD44v6 and CD44v9 (20) while normal bone marrow plasma cells do not express these isoforms. Clinically, the expression of isoform CD44v9 by myeloma plasma cells is correlated with adverse prognosis. In keeping with the unique expression of CD44 isoforms, Crainie et al. have shown that myeloma plasma cells express unique isoforms of RHAMM not found in healthy donor B cells 21. In addition to a full length isoform of RHAMM, myeloma plasma cells express RHAMM-48, which has a deletion of 48 bp, and RHAMM-147, which has a deletion of 147 bp. Presently however, the role of these isoforms in hyaluronan-binding is unknown. Interestingly, RHAMM is also expressed on a subset of myeloma bone marrow fibroblasts. The expression of RHAMM is confined to the intracellular compartment of cultured bone marrow mesenchymal stromal cells (Fig. 5), with no cell surface membrane expression detected by either fluorescence microscopy or flow cytometry. As with RHAMM expression in myeloma plasma cells, the expression of intracellular RHAMM is distinct to myeloma bone marrow fibroblasts and is not found in healthy donor fibroblasts nor bone marrow fibroblasts derived from patients with chronic lymphocytic leukemia, lymphoma, acute myeloid leukemia or hairy cell leukemia (Fig. 5). At this time, the function of intracellular RHAMM in hyaluronan binding, or the particular isoform of RHAMM expressed by myeloma mesenchymal stromal cells is not yet defined.
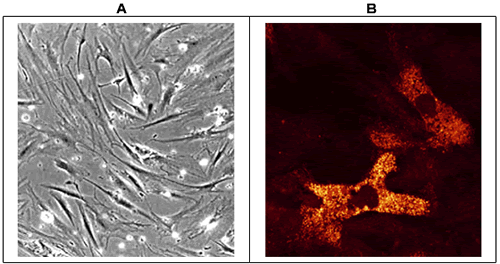
Fig. 5 Expression of RHAMM in Myeloma Bone Marrow Mesenchymal Cells
Myeloma-derived bone marrow mesenchymal stromal cells were grown to sub-confluency in α-MEM supplemented with 10% FBS and culture confluency determined by light microscopy (10x objective) [A]. Monolayers were permeabilized in 70% ethanol at 4°C and fixed in 1% formalin prior to staining for distribution of RHAMM using the anti-RHAMM mAb 3T3.5, followed by incubation with anti-mouse Ig conjugated to phycoerythrin. Stained cells were visualized and immunofluorescent images captured by laser confocal microscopy (40x objective). A subset (20%) of myeloma mesenchymal stromal cells expressed intracellular RHAMM as shown in [B], although CD44 was undetectable on these same cells (not shown). Images 4A and 4B were not derived from identical cultures.
We have shown that CD19+ peripheral blood B cells adhere to bone marrow stromal cell monolayers in a CD44-dependent manner. This adhesion was reduced (by 30%) when myeloma peripheral blood B cells, but not the stromal cells, were treated with mAbs against CD44. Interestingly, mAbs against RHAMM failed to block the adhesion. Furthermore, treatment of the stromal cells, but not the peripheral blood B cells, with Streptomyces hyaluronidase also reduced myeloma B cell adhesion indicating that hyaluronan on the stromal cells participated in interaction with CD44 on myeloma B cells. Similar adhesion studies using CD44 isoform specific antibodies have shown that myeloma bone marrow plasma cell adhesion to bone marrow stromal cells is mediated through CD44v6 and CD44v9 expressed on the plasma cells 22. We likewise show that myeloma plasma cell lines adhere to bone marrow mesenchymal stromal cells derived from healthy donors and myeloma donors (Fig. 6), which can be partially blocked by hyaluronidase treatment of the bone marrow mesenchymal cells but not by treatment of the plasma cells. Interestingly, hyaluronan-mediated adhesion is the predominant adhesion mechanism of myeloma cells, providing further supportive evidence for an important role of hyaluronan-mediated mechanisms in this disease.
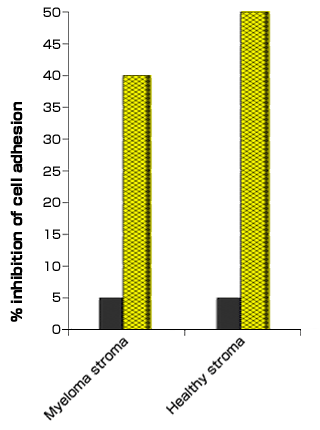
Fig. 6 Hyaluronan in Myeloma Plasma Cell Adhesion to Bone Marrow Mesenchymal Stromal Cells
Bone marrow mesenchymal stromal cells were derived from newly diagnosed, untreated myeloma patients or healthy donor bone marrow aspirates. Cultures were initiated by plating 1 x 106 cells/ml in α-MEM supplemented with 10% FBS. In some experiments, the mesenchymal stromal cell monolayers were pre-treated with 0.1 U/ml chondroitinase ABC (filled bars) or 40 TBU Streptomyces hyaluronidase (hatched bars), for 2 hrs, prior to addition of myeloma plasma cells labeled with 5 µM BCECF-AM (Molecular Probes, Eugene OR). Although chondroitinase ABC can also degrade hyaluronan, treatment of monolayers with chondroitinase ABC showed only 5% inhibition while treatment of monolayers with Streptomyces hyaluronidase resulted in 40-50% inhibition of adhesion.
Hyaluronan-dependent cell migration in myeloma: We have shown that myeloma B cells and leukemia plasma cells undergo cell migration on hyaluronan, but not on other matrix molecules including fibronectin or laminin. The migration of myeloma cells is accompanied by hyaluronan binding, and the binding of hyaluronan is inhibited by function blocking mAbs against both CD44 and RHAMM. However, only mAbs against RHAMM are able to inhibit migration on hyaluronan, indicating that RHAMM, but not CD44 mediates motility on hyaluronan 19. In a similar fashion, we show that myeloma bone marrow plasma cells bind hyaluronan in a CD44 and RHAMM dependent manner (Fig. 7a). Unlike circulating peripheral blood B cells and leukemia plasma cells derived from myeloma patients, myeloma bone marrow plasma cells do not migrate on hyaluronan substrate 19. However, we show that a small subset of myeloma plasma cells exhibit directed migration (transmigration) in response to fibronectin, but not to hyaluronan (Fig. 7b). In fact, the presence of hyaluronan appears to reduce migration below that observed on the control substrate BSA (Fig. 7b). Thus, although circulating and bone marrow resident B lineage cells in myeloma express CD44 and RHAMM receptors to equivalent levels and bind hyaluronan, the function of these receptors may be under cell-type specific regulation and mediate different cellular responses.
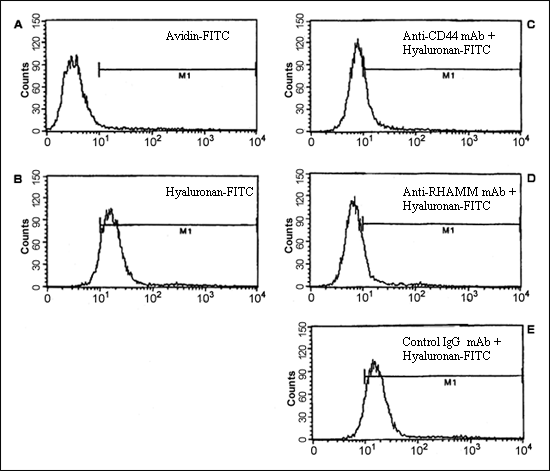
Fig. 7a Binding of Hyaluronan to Myeloma Plasma Cells
Plasma cells were analyzed for their ability to bind to hyaluronan-FITC (100 µg/ml) (B), in the presence or absence of 10 µg/ml (C) anti-CD44 (50B4) or (D) anti-RHAMM mAb (3T3.5). Avidin-FITC (100 µg/ml) and a non-specific IgG1 isotype control murine mAb (10 µg/ml) were used as controls (A and E respectively).
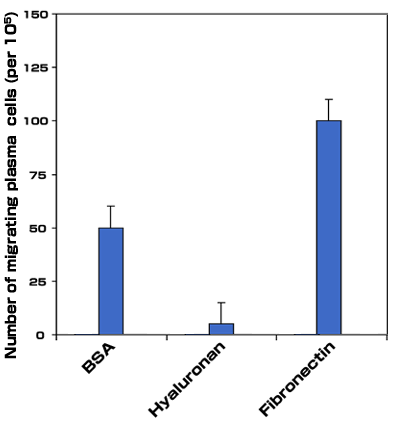
Fig. 7b Transmigration Response of Myeloma Cells in response to Hyaluronan and Fibronectin
To examine the transmigration of myeloma plasma cells, a modified Boyden chamber assay was used. 1 x 105 plasma cells were placed in the upper chamber of a transwell insert containing either 100 µg/ml hyaluronan or 20 µg/ml fibronectin and examined for their ability to migrate to the underside of filters after 1.5 hours of culture. Migrated cells were counted under inverted microscopy (magnification 20x). Results are expressed as the average of cells per field (minimum of 10 fields counted).
Hyaluronan-mediated signal transduction in myeloma cells: While hyaluronan has long been recognized as a component of the extracellular matrix, it is only fairly recently that hyaluronan has been implicated as more than an inert component. Current studies have described the induction of hyaluronan-receptor mediated signaling initiated in hematopoietic cells as a consequence of hyaluronan stimulation. The survival and proliferation of myeloma plasma cells may be partly dependent upon soluble factors as well as cell-cell and cell-matrix contacts. IL-6 is a well-characterized critical survival factor for myeloma cells and is produced mainly by the bone marrow mesenchymal stromal cells. Hyaluronan has demonstrated effects on the survival and growth of IL-6 dependent myeloma cells in conditions of IL-6 deprivation 23, conditions known to induce myeloma plasma cell apoptosis. In the presence of hyaluronan (5-80 µmg/ml), protection from apoptosis and an increase in cell proliferation was observed. This hyaluronan-mediated survival and proliferation effect was associated with the down-regulation of p27kip1 expression and the hyperphosphorylation of Rb, two molecules involved in cell-cycle progression through G1. Interestingly, the survival and proliferation effects of hyaluronan on myeloma plasma cell lines did not require CD44:hyaluronan interaction, but did involve signaling through the gp-130 IL-6 receptor subunit, suggesting the possibility of an hyaluronan-initiated autocrine feedback loop for IL-6 production and utilization.
Our group has demonstrated that hyaluronan stimulation of the plasma cell line ARH77, with hyaluronan concentrations that promote myeloma cell migration, results in the activation of Raf-1 and MAP kinases (Table 2). The activation of Raf-1 kinase was rapid and reproducible, with phosphorylation of MAP kinase kinase-GST occurring as early as 3 minutes and declining to base levels by 15 minutes. The increase in Map kinase activity ranged between 2-10 fold (Table 2). Furthermore, hyaluronan stimulation resulted in the up-regulation of IL-1β mRNA, which was inhibitable by the MEK inhibitor PD98059 (Fig. 8) indicating that IL-1β up-regulation occurs through a signal transduction pathway involving MEK. The production of IL-1β by myeloma plasma cells is of significance to the pathophysiology of the disease. IL-1β is a potent osteoclast activating factor and plays a role in the development of lytic bone lesions, a common and devastating clinical feature in multiple myeloma. IL-1β also up-regulates cytokine production, including IL-6 which is a major cytokine crucial to myeloma cell growth. Thus, it could be speculated that in myeloma patients, hyaluronan expressed by the bone marrow mesenchymal cells may initiate a chain of events, as a consequence of interactions with hyaluronan receptors on the plasma cells, that may lead to up-regulation of cytokines involved in the manifestation of bone disease as well as in myeloma plasma cell growth.
Table. 2 Hyaluronan Stimulation Activates Raf-1 and MAP kinase in Myeloma Plasma Cells
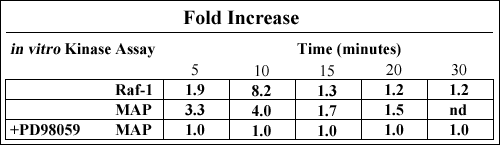
The myeloma cell line ARH77 was serum starved for a minimum of 4 hours prior to stimulation with 100 µg/ml hyaluronan and assay for kinase activity. In some experiments, cells were pre-treated with the MEK inhibitor PD98059 for 30 minutes prior to stimulation, and the inhibitor was maintained in the medium during hyaluronan stimulation. Cells were activated with hyaluronan at concentrations ranging from 10 – 100 µg/ml and maximal Raf-1 kinase was observed at 100 µg/ml. Thus, this concentration of hyaluronan was utilized in all subsequent experiments. Cell lysates were prepared; Raf-1 or MAP kinase immunoprecipitated, and in vitro kinase assays carried out using MEK-GST or MBP as substrates, respectively. In all experiments, fold increase was determined relative to the activity of each kinase at time 0. nd= not done
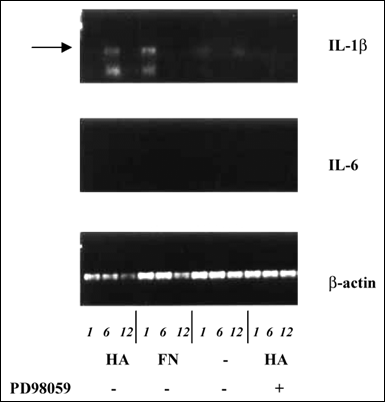
Fig. 8 Rt-PCR for IL-1β and IL-6 in Myeloma Plasma Cells after Hyaluronan Stimulation
Rt-PCR for IL-1β and IL-6 was performed on the myeloma ARH77 cells stimulated with either hyaluronan [HA] (100 µg/ml) or fibronectin [FN] (20 µg/ml) for 1, 6 and 12 hours. Unstimulated ARH77 cells express neither IL-1β nor IL-6. Rt-PCR products were run on a 1.5% agarose gel and stained with ethidium bromide. Shown is a representative of 3 independent experiments. The arrow highlights the IL-1β Rt-PCR product stimulated by HA at 6 hours and by FN at 1 hour. The stimulation of IL-1β message by hyaluronan is completely inhibited by the MEK inhibitor PD98059. Rt-PCR product for IL-6 was undetectable.
While the management of multiple myeloma and other hematological malignancies has focused on the eradication of the malignant cell, it is important to consider the impact of the marrow microenvironment on tumor response and normal cell function. In addition to the presence of malignant plasma cells, our data suggest that the bone marrow microenvironment in myeloma is abnormal possibly due to its exposure to tumor cells and/or chemotherapeutic agents. In particular, in multiple myeloma, hyaluronan synthesis may have prognostic value for a subgroup of patients. in vitro studies with myeloma plasma cells implicate hyaluronan in many functions associated with tumorigenesis, including elevated expression of hyaluronan receptors, enhanced cell migration and cell adhesion and altered cell signaling. Thus, the management of myeloma will depend not only on eliminating tumor cells but also on the re-establishment of the bone marrow microenvironment from one that supports tumor growth to one that supports normal cellular function. Increased insights into the role of hyaluronan in myeloma may eventually lead to the use of hyaluronan as a novel therapeutic target in myeloma. For instance, hyaluronan oligosaccharides may function to block migration, to block adhesion of plasma cells in the bone marrow, or to inhibit the establishment of cytokine loops established between the bone marrow mesenchymal cell and the myeloma plasma cell as a consequence of cell-cell interaction. Likewise, hyaluronan could serve a greater role as a prognostic tool. In that regard, the presence of different molecular weight hyaluronan, possibly as determined by HAS enzyme expression, could be used to identify patients with poor outcome. Based on the cited literature and data from our laboratory, we propose that in myeloma, hyaluronan synthesis and matrix organization in the bone marrow imparts a unique function to the bone marrow environment that may impact not only myeloma plasma cell growth and survival but also impact on normal bone marrow mesenchymal cell function.