Katsuhiko Yanagisawa
Department of Dementia Research, National Institute for Longevity Sciences
Deposition of amyloid β-protein (Aβ) is the most critical event in the development of Alzheimer's disease (AD). However, we are far from understanding the molecular mechanism underlying the aggregation and amyloid fibril formation of Aβ. Previously we reported a novel Aβ, which was characterized by its tight binding with the GM1 ganglioside, in AD brains in the early pathological stages 1. GM1 ganglioside-bound Aβ (GM1/Aβ) possesses unique characteristics, including an extremely high aggregation potential and an altered pattern of immunoreactivity. Thus, we hypothesized that Aβ adopts an altered conformation via binding with the GM1 ganglioside and then acts as a "seed" for amyloid fibril formation in brains predisposed to the development of AD.
Subsequent to our initial report, several investigators reported on the binding of Aβ with the GM1 ganglioside and the acquisition of an altered secondary structure by Aβ 2. Furthermore, it was also reported that GM1/Aβ was directly injurious to cellular membranes 3,4. It remains to be determined where and how Aβ binds with the GM1 ganglioside in AD brains. Currently, we propose that Aβ may bind with the GM1 ganglioside in lipid membrane domains called rafts or caveolae-like domains, where the concentrations of the GM1 ganglioside and cholesterol are higher than those in any other cellular membranes, and that the GM1 ganglioside may potentially alter the secondary structure of Aβ like a molecular chaperone, as was discussed for other conformational diseases including prion diseases and CAG repeat diseases.
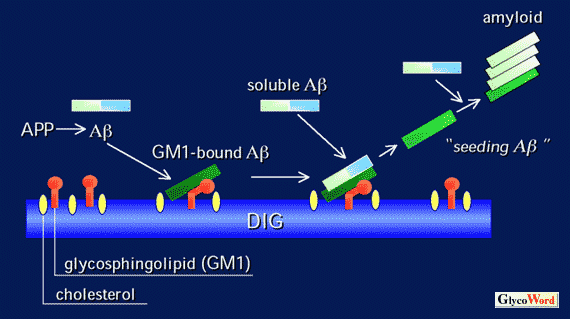
Fig. Hypothetical mechanism of the formation of GM1/Aβ and amyloid fibril
DIG: detergent-insoluble glycosphingolipid-rich domain