
Vincent C. Hascall
Department of Biomedical Engineering (Wb3), Lerner Research Institute, Cleveland Clinic Foundation, Cleveland, Ohio 44195
Described in Biosketch of Editors

Torvard C. Laurent
Dr. Torvard Laurent received his Doctor of Medicine from the Karolinska Institute, Stockholm in 1958. After a 3 year fellowship from the Retina Foundation in Boston, Dr. Laurent established a research program at the University of Uppsala, where he attained a Professorship in 1966. He is currently Professor emeritus at this institution and Science Secretary for the Wenner-Gren Foundations, Stockholm. Dr. Laurent has conducted pioneering, internationally recognized research throughout his career on the chemistry of connective tissues, most notably on the physical and physiological properties, and medical applications of hyaluronan. His numerous honors include: Deputy Chairman, Swedish Medical Research Council, 1973-77; Chairman, Swedish Biochemical Society, 1973-76; President, Royal Swedish Academy of Sciences, 1991-94; and Chairman, Council of the Nobel Foundation, 1994-present. His busy schedule includes responsibility for organizing international conferences for the Wenner-Gren Foundations, where he recently organized and edited one on the 'Structure, Biology and Medical Applications of Hyaluronan'.
1In 1934, Karl Meyer and his assistant, John Palmer, described a procedure for isolating a novel glycosaminoglycan from the vitreous of bovine eyes [1]. They showed that this substance contained an uronic acid and an aminosugar, but no sulfoesters. In their words: 'we propose, for convenience, the name "hyaluronic acid", from hyaloid (vitreous) + uronic acid.' This marked the birth announcement for one of nature's most versatile and fascinating macromolecules. Today, this macromolecule is most frequently referred to as 'Hyaluronan', reflecting the fact that it exists in vivo as a polyanion and not in the protonated acid form.
It would take an additional 20 years before Meyer's laboratory finally completed the work that determined the precise chemical structure of the basic disaccharide motif that forms hyaluronan [2]. During these years they showed that the uronic acid and aminosugar in the disaccharide are D-glucuronic acid and D-N-acetylglucosamine, and that they are linked together through alternating beta-1,4 and beta-1,3 glycosidic bonds, Fig. 1. Both sugars are spatially related to glucose which in the beta configuration allows all of its bulky groups (the hydroxyls, the carboxylate moiety and the anomeric carbon on the adjacent sugar) to be in sterically favorable equatorial positions while all of the small hydrogen atoms occupy the less sterically favorable axial positions. Thus, the structure of the disaccharide shown in Fig. 1 is energetically very stable.
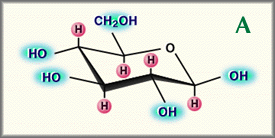

Fig. 1
Relationship between beta-D-glucose (A) and the repeat disaccharide of hyaluronan, D-glucuronic acid-beta-1, 3-N-acetylglucosamine-beta-1, 4 (B)
H ; axial hydrogens that contribute to the hydrophobic face
Hyaluronan synthase enzymes synthesize large, linear polymers of the repeating disaccharide structure of hyaluronan by alternate addition of glucuronic acid and N-acetylglucosamine to the growing chain using their activated nucleotide sugars (UDP - glucuronic acid and UDP-N-acetlyglucosamine) as substrates.1 The number of repeat disaccharides, n, in a completed hyaluronan molecule can reach 10,000 or more, a molecular mass of ~4 million daltons (each disaccharide is ~400 daltons). The average length of a disaccharide is ~1 nm. Thus, a hyaluronan molecule of 10,000 repeats could extend 10 オm if stretched from end to end, a length approximately equal to the diameter of a human erythrocyte. Fig. 2 shows an electron micrograph of a few intertwined hyaluronan molecules that have been deposited on a flat surface and rotary shadowed with heavy metal for contrast.
1 These enzymes and the mechanism of hyaluronan synthesis will be the subject of later articles in this series.
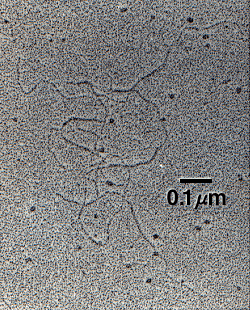
Fig. 2
The electron micrograph was kindly provided by Dr. Richard Mayne and Dr. Randolph Brewton, University of Alabama at Birmingham.
In a physiological solution, the backbone of a hyaluronan molecule is stiffened by a combination of the chemical structure of the disaccharide, internal hydrogen bonds, and interactions with solvent. The axial hydrogen atoms (indicated in Fig. 1B) form a non-polar, relatively hydrophobic face while the equatorial side chains form a more polar, hydrophilic face, thereby creating a twisting ribbon structure.2 Consequently, a hyaluronan molecule assumes an expanded random coil structure in physiological solutions which occupies a very large domain, Fig. 3. The actual mass of hyaluronan within this domain is very low, ~0.1% (wt/vol) or less when the macromolecule is present at a very dilute concentration in saline. This means that the domains of individual molecules would overlap each other at concentrations of 1 mg hyaluronan per ml or higher.
2 See article 2 by John Scott.
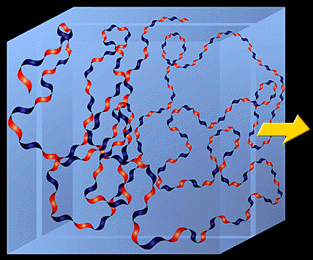
Fig. 3 Model of hyaluronan ribbon in a 3-dimensional domain.
The light blue box represents the domain of the molecule in solution. The alternating blue and red strand represents the ribbon structure with blue (hydrophilic) and red (hydrophobic) faces.
The slice is represented in Fig. 4.
The domain structure of hyaluronan has interesting and important consequences. Small molecules such as water, electrolytes and nutrients can freely diffuse through the solvent within the domain. However, large molecules such as proteins will be partially excluded from the domain because of their hydrodynamic sizes in solution. As shown in Fig. 4, the hyaluronan network in the domain allows less and less space for other molecules the larger they are. This leads both to slower diffusion of macromolecules through the network and to their lower concentration in the network compared to the surrounding hyaluronan free compartments. Interestingly, the hyaluronan chains are constantly moving in the solution, and the effective 'pores' in the network continuously change in size. Statistically, all sizes of pores can exist, but with different probabilities. This means that in principle, all molecules can pass through a hyaluronan network, but with different degrees of retardation depending on their hydrodynamic volumes.
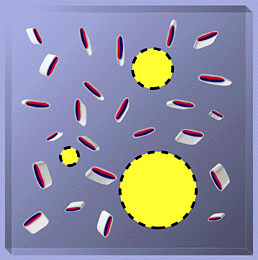
Fig. 4 Vertical slice from Fig. 3 illustrating average pore size and partial exclusion of large molecules.
The red and blue are tracings of regions in Fig. 3 representing portions of the hyaluronan backbone in the representative slice. The fuzzy halo (shading) around the hyaluronan fragments would be the volume of the slice inaccessible to a diffusing molecule. The 3 circles of different size represent areas available to diffusing molecules. The smallest would have access to most of the volume not occupied by hyaluronan while the largest would have access to only the place it is located and would clearly have a harder time moving through the hyaluronan domain (Fig. 3) than the smaller ones.
The pK of the carboxyl groups on the glucuronic acid residues is 3-4, depending on ion conditions. At pH 7, then, these groups are predominantly ionized, and the hyaluronan molecule is a polyanion that has associated, exchangeable cation counterions to maintain charge neutrality. Directional flow of electrolyte through such a polyanionic domain can lead to sufficient charge separation to create a streaming potential.
Hyaluronan is present in all vertebrates, perhaps arising in animals with notochords. It is also present in the capsule of some strains of Streptococci that quite likely pirated the enzymatic machinery for its synthesis from vertebrate hosts. Hyaluronan is a major constituent of the extracellular matrices in which most tissues differentiate.3 It is also an essential component of many extracellular matrices in mature tissues. In some cases, hyaluronan is a major constituent; as, for example, in the vitreous of the human eye (0.1-0.4 mg/g wet weight), or in synovial joint fluid (3-4 mg/ml), or in the matrix produced by the cumulus cells around the oocyte prior to ovulation (~0.5 mg/ml),4 or in the pathological matrix that occludes the artery in coronary restenosis.5
In others, while representing less of the mass of the tissue, hyaluronan serves as an essential structural element in the matrix. For example, hyaluronan is present at ~1 mg/g wet weight in hyaline cartilages, enough to fill the tissue volume in the absence of other constituents. However, aggrecan, the large chondroitin sulfate proteoglycan, is present at a much higher concentration (25-50 mg/g wet weight), and hyaluronan retains aggrecan molecules in the matrix through specific protein-hyaluronan interactions which mask the hyaluronan backbone.6 Hyaluronan is less concentrated in the matrix of other connective tissues, such as those surrounding smooth muscle cells in the aorta and fibroblasts in the dermis of skin. Like cartilage however, hyaluronan forms a scaffold for binding large chondroitin sulfate proteoglycans in the matrices of these tissues.
The largest amount of hyaluronan (7-8 g per average adult human, ~50% of the total in the body) resides in skin tissue, where it is present in both the dermis (~0.5 mg/g wet tissue) and the epidermis (~0.1 mg/g wet tissue). Interestingly, while dermis consists primarily of extracellular matrix with a sparse population of cells, the epidermis is the reverse; the keratinocytes fill all but a few percent of the tissue. Thus, the actual concentrations of hyaluronan in the matrix around the cells in the epidermis (estimated to be 2-4 mg/ml) is an order of magnitude higher than in the dermis (estimated to be ~0.5 mg/ml).7 The matrix around keratinocytes, then, may have a hyaluronan concentration as high as that in umbilical cord (~4 mg/ml) considered to be the mammalian tissue with one of the highest concentrations. Interestingly, rooster comb, a specialized piece of skin, has even higher amounts of hyaluronan (up to 7.5 mg/ml).
3 The role of hyaluronan in tissue morphogenesis will be the subject of a later article in this series.
4 See article 3 by Antonietta Salustri and Csaba Fulop.
5 The role of hyaluronan in formation of the arterial matrix in restenosis will be the subject of a later article in this series.
6 The role of hyaluronan for cartilage structure and function will be the subject of a later article in this series.
7 The role of hyaluronan in the epidermis will be the subject of a later article in this series.
The metabolism of hyaluronan is very dynamic. Some cells, such as chondrocytes in cartilages, actively synthesize and catabolize hyaluronan throughout the lifetime of the tissue. Synthesis is usually balanced by catabolism, thereby maintaining a constant concentration in the tissue. Metabolic studies have shown that the half life of a hyaluronan molecule in cartilage is normally 2-3 weeks. Keratinocytes in epidermis are another example of cells that actively synthesize and catabolize hyaluronan. In this case, the half life of a hyaluronan molecule is surprisingly short, less than a day.
Sometimes cells either predominantly synthesize or they catabolize hyaluronan. For example the cells in the dermis actively synthesize more hyaluronan than they catabolize. A large proportion of the hyaluronan molecules escape from this tissue only to be rapidly captured by receptors on reticulo-endothelial cells in lymph nodes and in the liver which internalize them for subsequent catabolism in lysosomes. The half life of a hyaluronan molecule in the blood is very short, only a few minutes. Tissues in joints, such as the lining cells of the joint capsule of the knee, synthesize hyaluronan and release it into the synovial fluid, where it becomes a major component that contributes to the viscoelastic properties of the fluid. Also, the synovial fluid drains through the lymphatics before entry into the bloodstream. Reticulo-endothelial cells lining the lymphatics actively remove almost 90 percent of the hyaluronan before the remainder reaches the vascular system. It has been estimated that almost one-third of the total hyaluronan in the human body is metabolically removed and replaced during an average day.
The concentration of hyaluronan in tissues is often higher than would be expected if individual molecules maintained their expanded domain structures. In many cases the hyaluronan is organized into the extracellular matrix by specific interactions with other matrix macromolecules. However, high molecular weight hyaluronan at high concentration in solution (for example, 5 million daltons at concentrations above 0.1 mg/ml) can also form entangled molecular networks through steric interactions and self association between and within individual molecules. The latter can occur when a stretch of the hydrophobic face of the ribbon structure of the backbone interacts reversibly with the hydrophobic face on a comparable stretch of hyaluronan on another molecule or in a different region of the same molecule. Such networks exhibit different properties than would isolated hyaluronan molecules. They can resist rapid, short-duration fluid flow through the network, thereby exhibiting elastic properties which can distribute load or shear forces within the network, Fig. 5. On the other hand, slow fluid flow of longer duration can partially separate and align the molecules, allowing their movement and exhibiting viscous properties. Procedures for introducing covalent cross-links in hyaluronan matrices have been developed to create stable networks and semi-solid materials exhibiting pronounced viscoelastic properties. 8
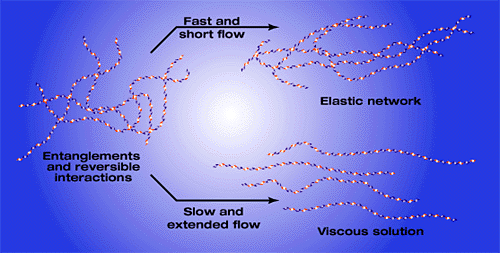
Fig. 5 Model demonstrating the viscous and elastic properties of hyaluronan solutions.
8 Cross-linked hyaluronan matrices and their applications will be the topic of a future article in this series.
Appropriately, the first medical application of hyaluronan for humans was as a vitreous supplement/replacement during eye surgery in the late 1950s. The hyaluronan used was isolated initially from human umbilical cord, and shortly thereafter from rooster combs in a highly purified and high molecular weight form. This latter preparation, now sold under the trade name of Healon (Pharmacia), is currently widely used for ophthalmic viscosurgery and in other forms of surgery, as is Opegan (Seikagaku), a hyaluronan product also prepared from rooster comb. Another hyaluronan product, Artz (Seikagaku), was developed for use as a supplement in the synovium of osteoarthritic joints, and a covalently cross-linked form of hyaluronan, Synvisc (Biomatrix), with more pronounced viscoelastic properties, is also being used for the same purpose.
We hope that this inaugural article in "The Science of Hyaluronan Today", will provide an overview and background information that you will find useful for understanding and appreciating the multi-faceted world of hyaluronan research that will unfold in the subsequent articles of this series.