
Kiyonori Nagaoka
Nara Institute of Science and Technology (NAIST), Graduate School of Science and Technology, Institute for Research Initiatives
He enrolled in April 2020 and completed the Master's program at Nara Institute of Science and Technology (NAIST), Graduate School of Science and Technology, in March 2022 (Master of Engineering).

Hiroaki Yoshida
Nara Institute of Science and Technology (NAIST), Graduate School of Science and Technology, Institute for Research Initiatives
In 2011, he completed his studies, received his PhD from the Graduate School of Engineering at Osaka University (Japan Society for the Promotion of Science DC1), and became a postdoctoral researcher at the Georgia Institute of Technology in the United States (funded by a Japan Society for the Promotion of Science Overseas Special Research Fellowship). In October 2013, he was a postdoctoral researcher at the Graduate School of Osaka University. In September 2014, he became an assistant professor at the Graduate School of Science, Shinshu University. In April 2019, he became an assistant professor at the Institute for Materials Science, Shinshu University Interdisciplinary Cluster for Cutting Edge Research (concurrent position). In November 2019, he became an assistant professor at Nara Institute of Science and Technology (NAIST).

Hiroharu Ajiro
Nara Institute of Science and Technology (NAIST), Graduate School of Science and Technology, Institute for Research Initiatives, Data Science Center, Medilux Research Center
In 2004, he completed his studies at the Graduate School of Engineering at Nagoya University (Japan Society for the Promotion of Science DC2). He received his PhD in Engineering. In 2004, he was a postdoctoral researcher at Cornell University in the United States as well as at the Japan Society for the Promotion of Science. In 2006, he became a project lecturer at Osaka University, Center for Integrated Research and Education in Clinical Biomedical Engineering. In 2011, he became a special associate professor at the same university. In 2015, he became a project associate professor at Graduate School of Science and Technology, Division of Material Science, Nara Institute of Science and Technology (NAIST). In April 2019, he became a professor at the same university. In July 2024, he became Director of Research and Promotion of Medilux Research Center at the same university (concurrent position).
In the electrospinning of agarose in solution, we explored and optimized the solvent conditions, and succeeded in preparing nanofibers with a diameter of 68 ± 33 nm in the presence of hexafluoroisopropanol/water (92.5/7.5, v/v) used as solvent. These results suggest that control of solvent hydrogen bonding is important for nanoscale materialization of agarose materials.
In recent years, there has been a great deal of interest in achieving Sustainable Development Goals (SDGs) and using natural compounds as substitutes for petroleum-derived materials. Research into issues related to natural compounds is opening up important new areas of technological innovation.
Agarose1–3 is a naturally occurring polymer that dissolves in water at high temperature and gels upon cooling. This gelation phenomenon is believed to be due to weak intermolecular hydrogen bonds, and reheating is believed to dissociate the bonds and restore the structure in solution4, but the mechanism behind this has not yet been elucidated. Because it is very safe, agarose is widely used in electrophoresis technology and as biomedical materials5, but its unique properties significantly limit its versatility and processability. In contrast, blended materials of agarose have also been investigated, and the development of materials in various forms, such as hydrogels and fibers blended with chitosan6, sponge-like materials7, and microcapsules8, for example, has been reported. From the above, the effective hydrogen bonds of agarose are useful for preparing bulk materials that solidify the entire system, not for preparing fine structures, particularly nano-sized structures, and this has not been fully explored so far. In other words, to date, there are no confirmed examples of nanomaterials made only from pure agarose.
Electrospinning is a process that uses positive high voltage to induce electrostatic repulsion between droplets of a polymer solution and involves spraying the solution onto a collector that is usually negatively charged. It is important to identify the optimal conditions necessary for the desired fiber morphology9. In addition, it has been reported that bead formation affects the physical properties of the fiber structure10. Bead formation can be avoided by lowering surface tension, using highly concentrated solutions and increasing the molecular weight of the polymer, which may increase fiber diameter11. It has also been shown that humidity affects bead formation, and it has been reported that smaller diameter fibers and no beads are produced when humidity is high12. Nanoscale fibers can have thousands of times more surface area compared to microscale fibers. This allows for efficient contact with molecules and particles that penetrate nonwoven fabrics13,14. Although a variety of materials, including petroleum-derived polymers15, dextran16, cellulose17, and alginic acid18 have been electrospun into nanoscale fibers, but there are very few examples of nanoscale particles electrospun from agarose.
Electrospun particles have been produced from carboxylated agarose19, acetylated agarose20, and blends of agarose with chitosan5, polyacrylic acid21, polyacrylamide22, polyvinyl alcohol23, alginate24, and curdlan25. However, to the best of our knowledge, electrospinning of pure agarose alone has not been successful. There has been only one report of the production of agarose fibers with a diameter of approximately 120 μm by wet spinning26, but to date no nano-sized fibers have been produced.
In this study, we aimed to obtain nanoscale agarose fibers by electrospinning agarose and controlling intermolecular hydrogen-bonding interactions through the use of solvents, additives, and changes in temperature and needle length (Figure 1).

Table 1 summarizes the electrospinning conditions for agarose solutions with various solvents and additives, and Figure 2 shows the results of laser microscopic observation. In order to achieve electrospinning of agarose, intramolecular hydrogen bonds must be broken and solvated. Therefore, a heater was attached to the syringe for the purpose of controlling hydrogen bond breakage and the amount of solvation due to hydrogen bonding, and a needle with a short pitch of 1.2 cm was used instead of the normally used 3.7 cm needle. First, trifluoroacetic acid (TFA) and dichloromethane (DCM) were mixed in a 7/3 (v/v) ratio (Table 1, entry 1), the solvent conditions used to electrospin a mixture of chitosan and agarose5, but only particles were observed (Figure 2a). Next, we investigated aqueous solution conditions and selected a solvent that was a mixture of water, ethanol, and hexafluoroisopropanol (HFIP).
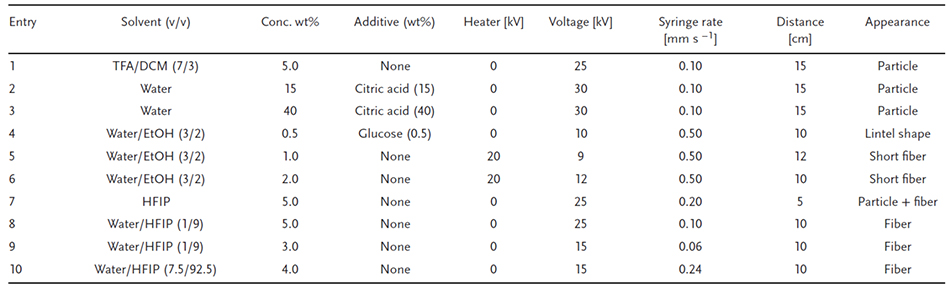
Citric acid and glucose were added to water and ethanol and used to prevent gelation at room temperature (Table 1, entries 2-4). However, when 0.5 wt% glucose was added to water/ethanol (3/2, v/v), only a lens-shaped particle was observed (Figure 2b). If additives are present in the solution, they will remain in the agarose spun from the discharge side even after the operation, which may make it difficult to control the amount of breakage of intermolecular hydrogen bonds and solvation due to hydrogen bonding. Therefore, a volatile solvent that can be removed during the spinning process was used. In this case, the syringe was heated to prevent gelation at room temperature and short fibers were formed (Table 1, entries 5, 6). Under conditions of 2.0 wt% agarose in water/ethanol (3/2, v/v), the diameter of the fibers was estimated to be 1.30 ± 0.37 μm (Figure 2bc). However, fiber formation was poorly reproducible, was only observed under these conditions, and failed to occur in all other samples. When the syringe was heated with a sheet-type heater, gelation at the needle tip was observed in most samples, making immediate injection virtually impossible. Overheating also caused evaporation of the ethanol solvent, complicating control. In this system, when a sheet-type heater was placed in the enclosed space of an electrospinning device and heated by passing an electric current through it, electric field interference resulting in equipment failure occurred due to the close proximity of the terminals. Therefore, HFIP was next selected as a solvent to break the intramolecular hydrogen bonds of agarose and dissolve it without heating.
When 5.0 wt% agarose was used in the HFIP, small amounts of fibers and particles were observed (Table 1, entry 7). Next, to change the hydrogen bond interactions within the agarose, water was mixed into HFIP and then the spinning conditions were adjusted. The use of water/HFIP (1/9, v/v), syringe flow rate set to 0.10 mm s-1, and spinning performed at a distance of 10 cm (Table 1, entry 8) resulted in increased amount of fibers compared to that when HFIP solvent alone was used (Figure 2d). Furthermore, when the syringe flow rate was slowed to 0.06 mm s-1 at 15 kV and agarose diluted to 3.0 wt% in water/HFIP was used (Table 1, entry 9), clearly transparent fibers were observed (Figure 2e). Here, we hypothesized that the viscosity of the agarose solution contributes significantly to fiber formation, and measured the relationship between viscosity and the water/HFIP ratio and agarose concentration. At all agarose concentrations (1.5, 2.0, 2.5, and 3.0 wt%), the highest viscosity in the water/HFIP solvent was observed at 7.5 vol% water (tested range 5–10 vol%). Furthermore, the solubility of agarose decreased when the water concentration was below 5 vol% and above 10 vol%. Finally, when 4.0 wt% agarose was used in water/HFIP (7.5/92.5, v/v) (Table 1, entry 10), very clear fibers were observed (Figure 2f).
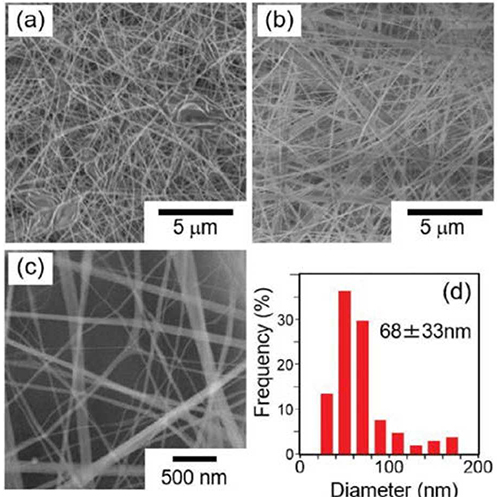
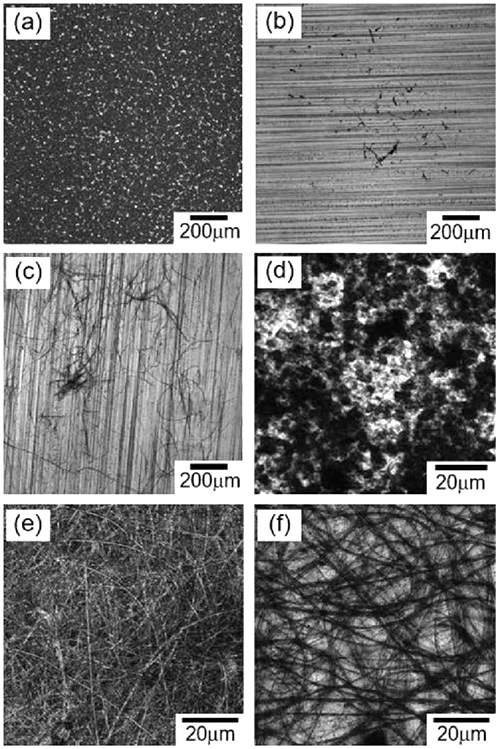
Agarose nanofibers were observed with a high-resolution field emission scanning electron microscope (FE-SEM). When agarose nanofibers were prepared in HFIP alone, large particles that appeared to be disorganized in some areas were formed, and the fibers were observed to be integrated (Figure 3a). However, when a high viscosity solution of 4.0 wt% agarose in water/HFIP (7.5/92.5, v/v) was used, fibers in good condition were observed (Figure 3b). When observed at high magnification, the smooth surface of the fibers was more clearly observed (Figure 3c). The diameter of the fibers was measured from the images and the results were compared and plotted as bar graphs, the diameter of the fibers was estimated to be 68 ± 33 nm, which is about 1/1200 of the previously reported agarose fiber diameter of 120 μm26, indicating that a novel and extremely fine material was obtained. Remarkably, it was possible to fabricate nanofiber sheets by stacking nanofibers (Figure 4a).
To characterize the agarose nanofibers, Fourier transform infrared spectroscopy (FT-IR) spectra of agarose nanofibers in range of 3500-3000 cm-1 (the hydrogen bonding region) were compared with those of agarose powder (Figure 4b). As a result, it was observed that the peak top of the stretching vibration of the hydroxyl groups in the agarose nanofibers were shifted to a higher wavenumber region (Figure 4b). This suggests that the binding of molecular hydrogen in agarose nanofibers is less than in agarose powder. To ensure the stability of the material, it may be necessary to reduce the number of unformed hydrogen bonds. We attempted to thermally crosslink agarose by heating at high temperatures in a humid environment, but no change was observed in the hydroxyl group peaks in FT-IR measurements. Reducing the number of unformed hydroxyl groups in hydrogen bonds remains a challenge for the future.
Finally, the swelling ratio (S.R.) of the agarose nanofiber as a gel fiber was estimated. S.R. is defined by the following formula (Formula A), where ws represents the weight of the equilibrium swollen fiber under high humidity conditions, and refers to the state in which the saturated humidity is reached by using a sufficient amount of water in a closed container at 25°C. wd is the weight of the dry fiber. The S.R. was about 15, which was lower than that of a typical bulk agarose gel, but showed a swelling degree almost equal to or greater than that of a neutral hydrogel.
S.R. = (ws - wd)/wd (A)
We developed a method to fabricate agarose nanofibers using electrospinning. The fiber diameter was 68 ± 33 nm, which is much finer than previously reported. It is believed that control of the amount of hydrogen bond formation and breakage and the use of low boiling point solvents are important in fabricating the nanofibers. Taking advantage of the large surface area provided by the naturally derived material, this new agarose fiber is expected to have a variety of applications in the future.。