
Eiji Hirano
Master’s Degree, Department of Chemistry and Biotechnology Engineering, Graduate School of Engineering, The University of Tokyo
Graduated from Yokohama National University in 2023 and earned his Master degree in the Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, in 2025. During his graduate studies, he conducted research on the development of supramolecular plastics at RIKEN (Wako, Saitama), where he received the RIKEN Baihou Prize for his work.

Takuzo Aida
Distinguished Professor, The University of Tokyo
Graduated from Yokohama National University in 1979 and received a Ph.D. from the Graduate School of Engineering, The University of Tokyo in 1984. After serving as Research Associate and Associate Professor at the Graduate School of Engineering, The University of Tokyo, he was appointed Professor in 1996 and has held his current position since 2022. He served as Project Director of the ERATO project (2000–2005) and has led a research group at RIKEN since 2009. His research focuses on innovative soft materials such as plastics derived from water and self-healing polymer glass. He has received numerous awards, including the Medal with Purple Ribbon and the Japan Academy Prize. He is an international member of the Netherlands Academy of Arts and Sciences, US Academy of Engineering, American Academy of Arts and Sciences, and Kao Executive Fellow.
Plastic waste has emerged as a pressing environmental challenge. Nevertheless, modern society continues to rely heavily on plastics for their affordability and convenience. Supramolecular polymers present a promising pathway toward addressing this issue. Our research seeks to develop “innovative polymer materials for the Sustainable Development Goals era” that display unique solid-state properties unattainable with conventional covalent polymers. In this review, we highlight a class of supramolecular plastics that not only exhibit outstanding mechanical performance but also demonstrate environmental responsiveness. In the presence of electrolytes, these materials can fully dissociate into their constituent monomers.
In modern society, plastics have become indispensable materials with applications spanning a wide range of fields. Plastics are composed of polymers—macromolecules consisting of numerous monomer units covalently linked through polymerization reactions—and the majority of these polymers are derived from fossil resources. However, once discarded, plastics rarely undergo complete degradation in the natural environment. Instead, through processes of weathering, they gradually fragment into fine particles with diameters below 5 mm, commonly referred to as “microplastics.” The accumulation of microplastics in the environment is a growing concern, as it poses potentially harmful effects on ecosystems and the global environment1. Recent studies have further revealed that microplastics accumulate not only in the natural environment but also within the human body2. Once ingested through food, water, or air, microplastics are transported via the bloodstream and have even been detected in the brain, raising serious concerns regarding their potential health impacts3.
As recognition grows that microplastics threaten not only the environment but also human health, there is an urgent need to fundamentally reconsider the concept of conventional plastics. Supramolecular polymers, assemblies in which monomeric components are held together by reversible noncovalent interactions, offer a promising platform for the design of next-generation sustainable plastics4,5. Unlike traditional polymers, supramolecular polymers can readily dissociate back into their constituent monomers in response to external stimuli. This unique property enables the realization of closed-loop recycling and reuse, without generating persistent plastic waste. Nevertheless, the reversibility of their noncovalent bonds has historically confined them to soft, rubber-like materials, long regarded as unsuitable replacements for conventional plastics in practical applications.
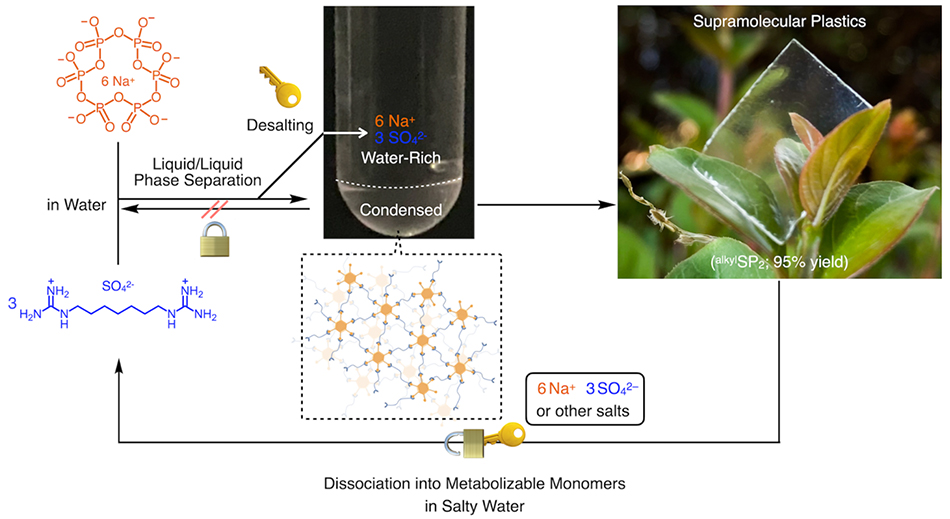
We have recently challenged this long-standing preconception by developing a new class of supramolecular plastics (SPs) whose dynamic behavior can be modulated by electrolytes6. Specifically, we combined guanidinium-based compounds with sodium hexametaphosphate (SHMP) or polysaccharides (Fig. 1), which undergo salt-bridging to form a robust polymeric network. Subsequent desalting via liquid–liquid phase separation (LLPS) suppresses the reversible dissociation of the network into its monomeric components. This unprecedented mode of supramolecular ionic polymerization has enabled us to achieve mechanical properties distinct from those of conventional supramolecular polymers. Moreover, by employing polysaccharides, we successfully obtained more deformable SPs, which could further be processed by 3D printing into complex structures.
Ultimately, we must confront a fundamental question: what kind of Earth will we leave to future generations? Preserving a beautiful and habitable planet for the children of tomorrow is a responsibility shared by all of us living today. The concept of SPs introduced in this study may represent an important step toward fulfilling that responsibility and building a truly sustainable future.
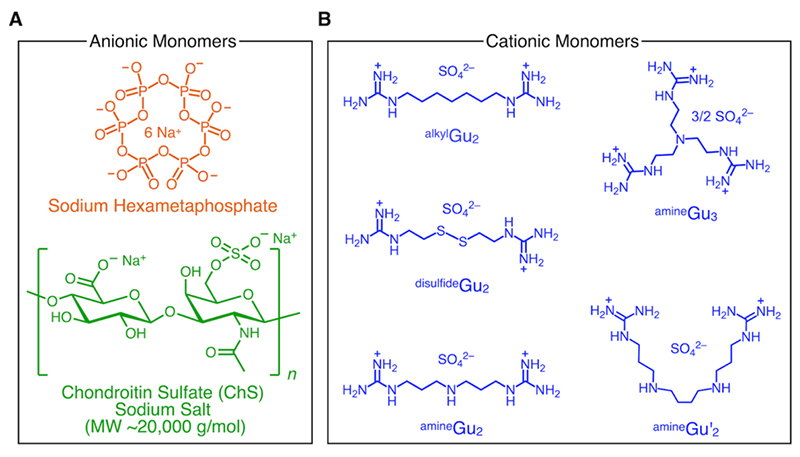
In this study, we present a new polymerization strategy for supramolecular polymers, which we term supramolecular ionic polymerization (Fig. 2). This approach harnesses ionic interactions between oppositely charged monomers in aqueous media to construct supramolecular polymer networks. A central question arises: how can inherently reversible, noncovalent bonds be transformed into strong, durable, plastic-like materials? The key lies in the synergy of salt bridges and LLPS.
Salt bridges, electrostatic interactions reinforced by hydrogen bonding, are among the strongest noncovalent interactions operative in aqueous systems7-9. We selected two ionic compounds that are both metabolizable and capable of forming such interactions. The first is SHMP, a compound widely employed as a food additive and soil conditioner10-12. The second is a multitopic guanidinium sulfate monomer, readily obtained in a single-step aqueous synthesis from its corresponding amine precursor13.
When combined in water, these compounds undergo multivalent crosslinking through salt-bridge interactions while simultaneously undergoing spontaneous LLPS. This process yields a condensed polymer-rich lower phase, while inorganic counterions originally associated with the two monomers partition into the water-rich upper phase. Crucially, this desalting step is indispensable: the counterions act as “keys” that would otherwise enable dissociation back to monomers. Their removal effectively “locks” the supramolecular assembly, stabilizing the network and suppressing its intrinsic reversibility. As a result, the process yields robust supramolecular polymers with mechanical properties far exceeding those of conventional systems. Drying the dense phase produced amorphous, colorless, and transparent SPs with an exceptionally high Young’s modulus of 17 GPa, well above that of commodity plastics (∼2 GPa)14.
Notably, this locking mechanism can be reversed by external electrolytes. In marine environments, for example, the introduction of ions disrupts the crosslinked network, triggering dissociation into monomers. Consequently, the material does not persist but instead undergoes biodegradative disappearance, thereby preventing the accumulation of microplastics.
In summary, by integrating salt-bridge formation with LLPS, we have established a new class of SPs that combine mechanical robustness with environmental degradability: properties previously thought to be mutually exclusive in supramolecular polymer systems.
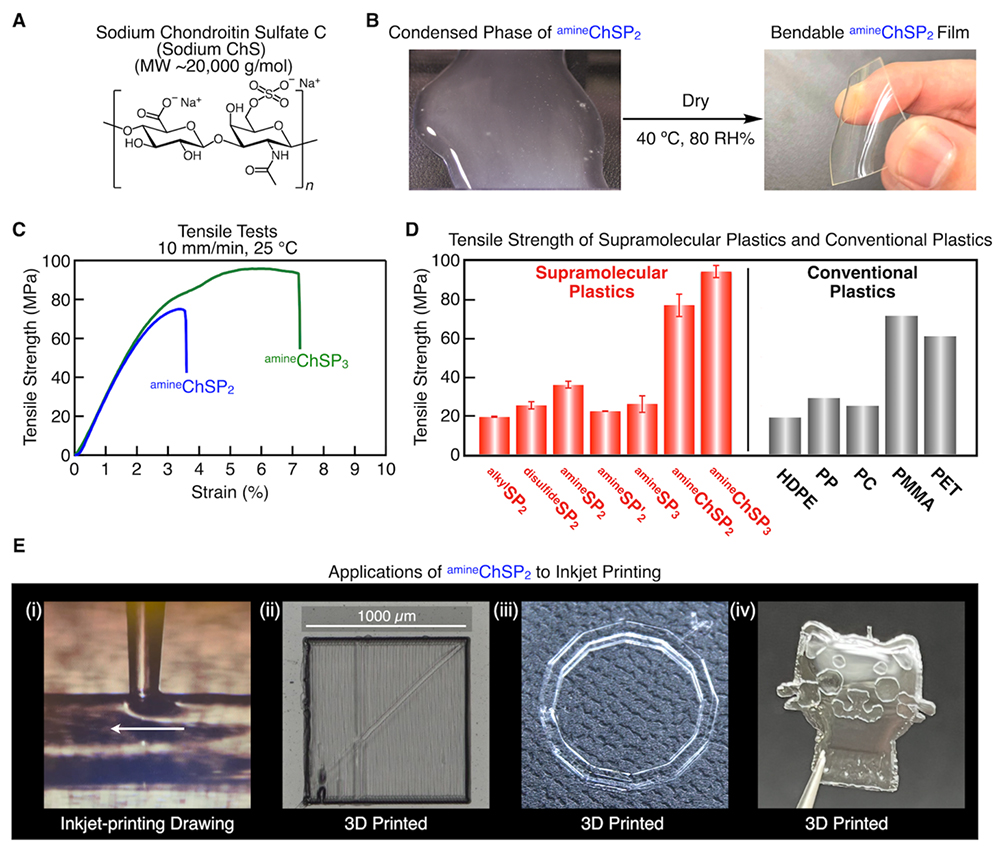
We next extended the LLPS-driven supramolecular polymerization strategy to natural polysaccharides as the oxyanion component. While SPs derived from SHMP exhibited a high Young’s modulus, they suffered from low tensile strength, a critical limitation for practical applications.
To overcome this drawback, we investigated the incorporation of polysaccharides to enhance the tensile properties of SPs. Polysaccharides are abundant, naturally occurring polymers with intrinsic biocompatibility and biodegradability. Their repeating monosaccharide units present diverse functional groups, depending on the biological source, some of which are capable of forming salt bridges with guanidinium monomers. These interactions are anticipated to modulate crosslink density and chain flexibility, thereby improving tensile strength.
Chondroitin sulfate (ChS) is a polysaccharide consisting of alternating D-glucuronic acid and N-acetyl-D-glucosamine units, variably substituted with sulfate groups. Predominantly found in the extracellular matrix and on cell surfaces as proteoglycans, ChS has been widely employed in osteoarthritis treatments, ophthalmic formulations, and dietary supplements15. Its anionic sulfate and carboxylate groups readily form salt bridges with guanidinium moieties, while its inherent biocompatibility allows seamless incorporation into SPs without compromising environmental sustainability.
The condensed phase was collected and dried in a humidity-controlled chamber at 40°C and 80% relative humidity for 24 hours, affording a colorless, transparent, free-standing film (amineChSP2). When a tritopic guanidinium monomer (amineGu3) was used, the resulting film (amineChSP3) exhibited further enhanced mechanical strength. Both films were readily bendable by hand, in sharp contrast to the brittle, glassy SPs. This flexibility is attributed to the reduced crosslinking density introduced by polysaccharide incorporation, as well as the plasticizing effect of water retained within the hydrophilic polysaccharide matrix. Tensile testing revealed high tensile strengths for both amineChSP2 and amineChSP3, with that for amineChSP3 reaching 93.6 ± 3.4 MPa. Comparative analysis with commodity plastics demonstrated that the mechanical performance of these supramolecular films rivals that of polyethylene terephthalate and poly(methyl methacrylate).
The coacervates obtained via LLPS exhibited sufficient viscosity to serve as printable inks. When extruded onto a hot stage at 30°C, the material could be patterned into microscale linear filaments, circular architectures, and even complex shapes such as a cat. These demonstrations indicate that this supramolecular plastic has an acceptable processability. By tuning viscosity through the selection of different monomers or polysaccharides, their post-printing mechanical integrity and shape fidelity can be further optimized. Such versatility underscores the potential of this class of materials for fabricating soft devices with intricate geometries, as well as for applications in advanced biomaterials.
The SPs developed in this study represent a promising pathway toward sustainable alternatives to conventional polymers. Their intrinsic dissociability and recyclability underscore their potential to alleviate environmental burdens while enabling closed-loop material use. Extending this design principle to other polysaccharides and renewable resources could further expand their applications, ranging from everyday commodities to advanced industrial materials. Beyond their practical significance, this work also bridges the divide between covalent polymer–based conventional plastics and noncovalent supramolecular polymers, offering new insights into the structure–dynamics relationships that underpin solid supramolecular materials.