
Akihide Yoshihara
Assistant Director, International Institute of Rare Sugar Research and Education, Kagawa University; Associate Professor, Faculty of Agriculture, Kagawa University
Dr. Yoshihara graduated from the Department of Bioresources Chemistry, Faculty of Agriculture, Kagawa University in 2004. After completing his master’s course at the Graduate School of Agriculture, Kagawa University in 2006 and his doctoral course at the United Graduate School of Agricultural Sciences, Ehime University in 2009, he was appointed an assistant professor at the Kagawa University Rare Sugar Research Center in 2009. Since 2017, he has been in his current position as an associate professor in the International Institute of Rare Sugar Research and Education, Kagawa University and as an associated professor in the Faculty of Agriculture, Kagawa University.
His research interests include applied enzymology and microbiology. Focusing on the production of new rare sugars using enzymes and microorganisms, he is fascinated by the power of enzymes and the potential of rare sugars.

Hiromi Yoshida
Associate Professor, International Institute of Rare Sugar Research and Education, Kagawa University; Associate Professor, Faculty of Medicine, Kagawa University
Dr. Yoshida graduated from the Department of Biotechnology, Faculty of Engineering, Tokyo University of Agriculture and Technology in 1994. After completing her master’s course at the Graduate School of Engineering, Tokyo University of Agriculture and Technology in 1996 and completing her doctoral program in biotechnology and obtaining a doctoral degree in engineering from the Graduate School of Engineering, Tokyo University of Agriculture and Technology in 1999, she was appointed an assistant professor in the Department of Biotechnology and Life Science, Faculty of Engineering, Tokyo University of Agriculture and Technology in 1999, and worked as a New Energy and Industrial Technology Development Organization (NEDO) fellow for the Marine Biotechnology Institute in 2001, as a post-doctoral researcher at the University of Groningen in 2002–2004, and as an associate professor for the Kagawa University Information Technology Center in 2004. Since 2007, she has been in her current position as an associate professor at the Basic Life Science Research, Faculty of Medicine, Kagawa University.
Her research interests include protein engineering and structural biochemistry. She is joyously working in rare sugar research with a team of colleagues working on development of new methods of rare sugar production through conducting structural analysis of various enzymes related to rare sugar production.

Susumu Mochizuki
Associate Professor, International Institute of Rare Sugar Research and Education, Kagawa University; Associate Professor, Faculty of Agriculture, Kagawa University
Dr. Mochizuki graduated from Cluster 3 (Chemistry), School of Engineering, Hiroshima University in 1999. After completing the first half of his doctoral course at the Graduate School of Advanced Sciences of Matter, Hiroshima University in 2001, he worked as a technical staff member for RIKEN Plant Science Center in 2001–2005 and as a Japan Society for the Promotion of Science research fellow (DC1) in 2005–2008. In 2008, he completed the second half of the doctoral course and obtained a doctoral degree in science from Hiroshima University. He then served as a post-doctoral researcher for the National Institute of Agrobiological Sciences in 2008–2014, as a post-doctoral researcher for the Faculty of Agriculture, Kagawa University in 2014–2016, and as an assistant professor for the International Institute of Rare Sugar Research and Education, Kagawa University in 2016. Since 2021, he has been in his current positions as an associate professor in the International Institute of Rare Sugar Research and Education, Kagawa University and as an associated professor in the Faculty of Agriculture, Kagawa University.
His research interests include microbial and plant genomics and molecular cell biology. Rare sugars are compounds that occur in very low abundances in nature. He aims to bring them into practical application by exploring the “general-purpose genes” of rare-sugar-producing plants and microorganisms and the metabolism genes responsible for rare sugar production and their actions.

Shiro Kato
Associate Professor, International Institute of Rare Sugar Research and Education, Kagawa University; Associate Professor, Faculty of Agriculture, Kagawa University.
Dr. Kato graduated from the Department of Applied Biosciences, Faculty of Agriculture, Nagoya University in 2004. After completing the first half of his doctoral course at the Nagoya University Graduate School of Bioagricultural Sciences in 2006, he left the Graduate School after earning credits for the second half of his doctoral course in 2009 and obtained a doctoral degree in agricultural sciences in 2010. He was appointed a researcher at the Nagoya University Graduate School of Bioagricultural Sciences in 2009, a post-doctoral fellow at the Organization for Research and Development of Innovative Science and Technology, Kansai University in 2013, and assistant professor at the International Institute of Rare Sugar Research and Education, Kagawa University in 2016. Since 2021, he has been in his current positions as associate professor at the International Institute of Rare Sugar Research and Education, Kagawa University and as an associate professor at the Faculty of Agriculture, Kagawa University.
His research interests include biochemistry and microbiology. He is exploring and analyzing the functions of new enzymes that are potentially involved in the metabolism of rare sugars.
In the second article of the Rare Sugars Series, rare sugars were shown to be producible using various enzymes (e.g., aldose isomerase, ketose 3-epimerase, polyol dehydrogenase), and the Izumoring was introduced as a key element in a strategy for comprehensive production of rare six-carbon hexoses by combining their enzymatic reactions. In this article, we describe the use of ketose 3-epimerase for producing the rare sugar D-allulose (=D-psicose) from naturally abundant D-fructose, and the use of L-rhamnose isomerase for producing the rare sugar D-allose from the thus-produced D-allulose. Currently, there are no “rare sugar producing enzymes” that catalyze the production of rare sugars, that is, sugars found in low abundance in a natural environment. However, enzymes capable of catalyzing the conversion of non-rare sugars to rare sugars exist in nature. We characterized some microbial enzymes that we successfully used to produce rare sugars, and the results are reported below.
As described in the second article, D-tagatose 3-epimerase, a new enzyme, is capable of catalyzing the epimerization of the C3-position hydroxyl group in ketopentoses (five-carbon sugars) and ketohexoses (six-carbon sugars). This enzyme is produced by Pseudomonas cichorii strain ST-24 (P. cichorii ST-24), which was discovered in soil in 1991 via a process of producing rare sugars from galactitol using P. cichorii ST-24. A study of the bacterial cell reaction of this strain using galactitol as the substrate revealed an accumulation of an unidentified product. Attempts to identify the product showed that this strain oxidizes galactitol to D-tagatose and converts the intermediate to the final product D-sorbose1,2. Further investigations demonstrated the involvement of the ketose 3-epimerase produced by P. cichorii ST-24 in the conversion of D-tagatose to D-sorbose. Furthermore, this enzyme was purified, examined for enzymological properties, and found to be most reactive with D-tagatose. Hence, it was named D-tagatose 3-epimerase (D-TE) and shown to catalyze the reversible epimerization of the C3-position hydroxyl group in ketopentoses and ketohexoses2 (Fig. 1). Because of that, we were able to produce a wide variety of rare sugars using this enzyme, which in turn led to the conceptualization of the Izumo-ring’s role in a production strategy for rare sugars3.
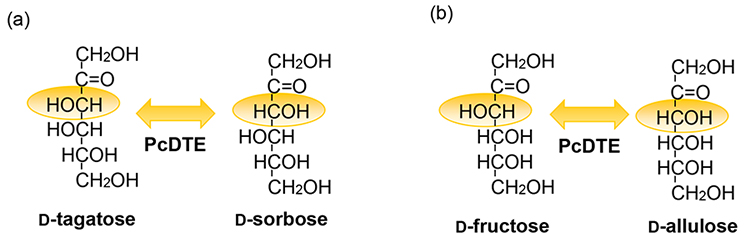
D-TE from P. cichorii ST-24 was found to be capable of acting on all forms of ketopentose and ketohexose, making it possible to mass-produce the rare sugar D-allulose. D-allulose is a C3 epimer of D-fructose, a naturally abundant sugar, and because the efficiency of D-fructose’s conversion to D-allulose by D-TE is approximately 25%, it is possible to mass-produce D-allulose. Early in the development of a mass-production strategy, a method of removing D-fructose by an assimilation reaction with baker’s yeast was used to fractionally purify D-allulose from a D-fructose:D-allulose=75:25 reaction liquor at equilibrium following the reaction. Around the year 2000, productivity for pure D-allulose was improved with the use of the pseudo-mobile chromatographic separator4. In addition, the newly acquired capability of mass-production of D-allulose led to the production of new rare sugars using the thus-produced D-allulose as the substrate.
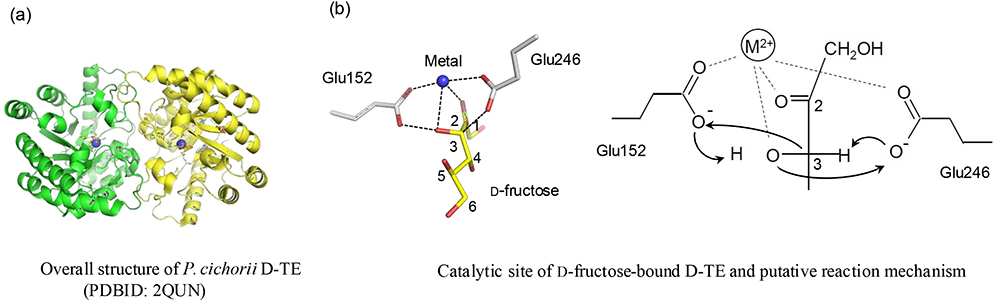
X-ray structure of D-TE, which is capable of catalyzing D-allulose production, showed that D-TE had a homodimer-forming subunit with triosephosphate isomerase (TIM) barrel structure (Fig. 2 (a), Fig. 3)5. The substrate complex structure supports a mechanism of D-TE catalysis via formation of a cis-endiolate intermediate. At the active site binding D-fructose as the substrate, divalent metal ions form a coordinate bond between amino acid residues, including two catalytic residues (Glu152 and Glu246), and the 2-position carbonyl oxygen and 3-position hydroxy group of D-fructose. When the two catalytic residues form a sandwich with the substrate, Glu246 extracts the proton from the C3 position of the substrate to form a cis-endiolate intermediate, and then Glu152 extracts the proton from the O3 position of the substrate. Simultaneously, a proton moves from Glu246 to the O3 of the substrate, and Glu152 donates a proton to the C3 position. In this reaction mechanism, the Glu of reversibly reactive catalytic residues are always ionized, and protons at C3 and O3 are exchanged, so we have proposed the C3-O3 proton exchange mechanism (Fig. 2 (b))5. In addition, in the substrate complex structure bound to the essential substrate D-tagatose, like in the D-fructose-bound structure, the 2-, 3-, and 1-positions of the substrate, which bind to metal ions and have catalytic activity, are strongly recognized by the enzyme, whereas the 4- and 5-positions of the substrate are weakly recognized. This finding supports the identity of D-TE as an enzyme of broad substrate specificity that is capable of utilizing not only D-tagatose but also all forms of ketohexose and ketopentose as substrates, thus explaining how D-TE led to the conceptualization of the Izumoring and became the key enzyme among the rare sugar production enzymes capable of catalyzing D-allulose production from D-fructose.
D-TE from P. cichorii became the basic enzyme for rare sugar production and the starting point of the Izumoring strategy used to produce the rare sugar D-allulose from naturally abundant D-fructose, and is now considered a member of the first family of ketose 3‐epimerases. Thereafter, D-allulose 3-epimerase (D-AE), a member of the second family of ketose 3‐epimerases, was discovered to exhibit higher activity when D-allulose is substrate. Since then, many D-AEs from a wide variety of microorganisms (e.g., Agrobacterium tumefaciens6; Clostridium bolteae7; Clostridium cellullyticum8; Dorea sp.9; Ruminococcus sp.10; Treponema primitia11) have been reported one after another. Since D-TEs and the early reported D-AEs usually have low heat stability, screenings were conducted for enzymes with higher heat stability; L-ribulose 3-epimerase, a member of the third family of ketose 3‐epimerases, which have high heat stability and relatively high enzyme reactivity with D-allulose, was also reported as capable of catalyzing the production of D-allulose from D-fructose (Arthrobacter globiformis12,13; Methylomonas sp.14; Labedella endophytica15). Highly stable D-AEs (e.g., DaeM from metagenomic DNA16) have been obtained from microorganisms including Pirellula sp.17, Arthrobacter psychrolactophilus18, Clostridia bacterium19, and Thermogutta terrifontis20, and enzymatically improved D-AEs have been obtained from Dorea Sp.21, Clostridium bolteae22, Halanaerobium congolense23, and Agrobacterium sp.24. These ketose 3‐epimerases form homodimers or homotetramers with different substrate specificity, and their subunits have TIM barrel structures (Fig. 3). These subunits have very similar structures, with amino acid residues, including catalytic residues, that are well conserved at the major active sites.
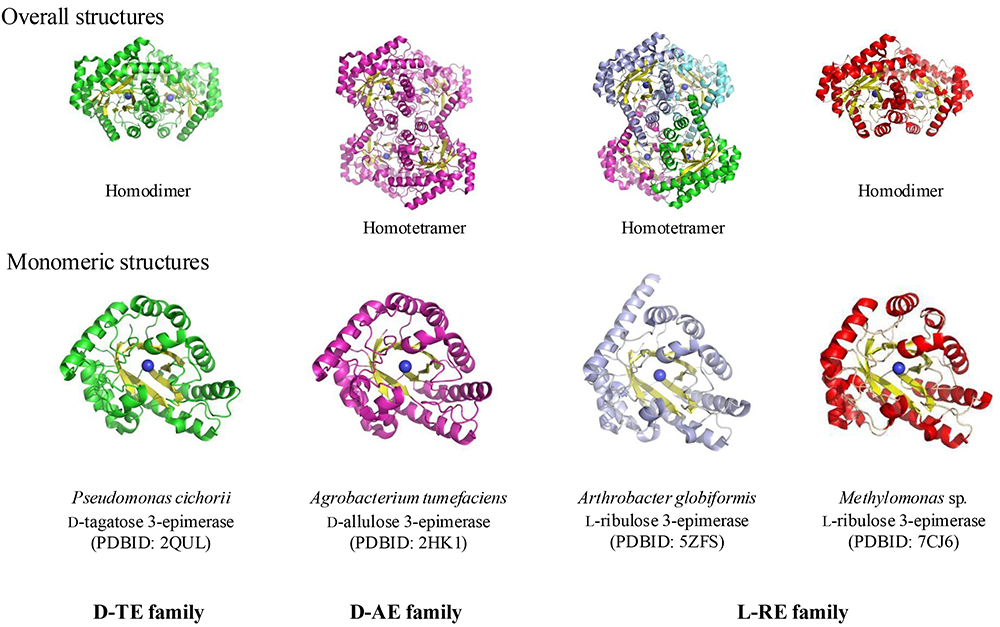
As described above, emphasis is placed on the characteristics (specificity toward D-allulose) of the ketose 3-epimerases used to production of D-allulose; recently, D-AEs were identified as the main enzymes used to D-allulose production. Members of the ketose 3-epimerase family with known detailed crystal structures include A. tumefaciens D-psicose 3-epimerase (AtDPE)25, Pseudomonas cichorii D-tagatose 3-epimerase (PcDTE)5, C. cellulolyticum D-psicose 3-epimerase (CcDPE)26, Pseudomonas cichorii D-TE C66S (PcDTEC66S)27, Arthrobacter globiformis D-AE (AgDAE)13, L-ribulose 3-epimerase from Methylomonas sp. (MetLRE)14, D-AE from Clostridia bacterium (CloDAE)28, Sinorhizobium fredii D-AE (SfDAE)29, Agrobacterium sp. D-AE24, and Kroppenstedtia eburnean D-TE (KeDTE)30. Further improvements of enzymatic properties including thermal stability of these ketose 3-epimerases are expected.
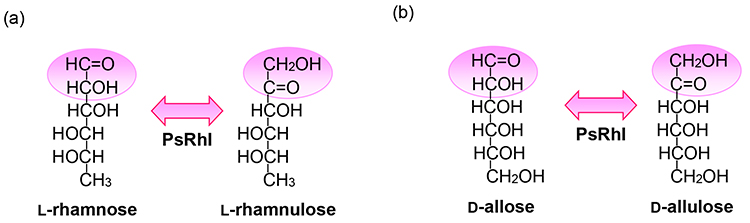
Mass-production of D-allulose has enabled us to produce new rare sugars using the thus-obtained D-allulose as the starting material. One such approach is to convert D-allulose to D-allose using an Izumoring strategy, representing the production of D-allose using L-rhamnose isomerase (L-RhI). Derived from a wide range of microorganisms, including E. coli, L-RhI had been reported to catalyze the isomerization between L-rhamnose and L-rhamnulose, and Izumori et al. reported that L-RhI derived from Pseudomons sp. strain LL172 (later identified as L-RhI from Pseudomonas stutzeri: PsL-RhI) was capable of producing D-allose from D-allulose31 (Fig. 4). Pseudomonas sp. LL172, a microorganism isolated from soil, produces L-RhI inductively in the presence of L-rhamnose. By subjecting this strain to shaking culture in the presence of L-lyxose, which cannot be metabolized by the strain, we succeeded in acquiring a L-lyxose-metabolizing mutant strain, which was shown to express L-RhI in a constitutive manner. Additionally, when purified by various column chromatographies and examined for enzymological properties, PsL-RhI was found to have broad substrate specificity and to be capable of reacting with not only L-rhamnose but also L-lyxose, L-mannose, D-gulose, D-ribose, D-allose, and L-talose. Furthermore, attempts to mass-produce D-allose using PsL-RhI have been successful in mass-producing D-allose from D-allulose using a pseudo-mobile chromatographic separator.
The structure of an L-RhI (specifically the L-RhI derived from E. coli [EcL-RhI]) was reported for the first time by Korndörfer et al.32, and its catalytic reaction mechanism was elucidated. Thereafter, X-ray crystallographic analysis of PsL-RhI, an enzyme used to produce rare sugars, clarified the structure of the enzyme-substrate (rare sugar) complex33. Like EcL-RhI, PsL-RhI was found to have a subunit with a TIM barrel structure composed of a homotetramer (Fig. 5 (a)) and an active site bound by two metal ions (M1 and M2) (Fig. 5 (b)). These two metal ions are known to stabilize enzyme binding to the substrate (M1) and to contribute to the catalytic reaction (M2). The substrates O1, O2, and O3 form coordinate bonds with the metal ions and are strictly recognized by enzymes as well. The catalytic reaction mechanism of L-RhI is a 1,2-hydride-shift mechanism in which between the 1- and 2-positions of the substrates aldose and ketose undergo an isomerization reaction under the influence of catalytic water. No amino acid residue is present to act as an acid-base catalyst in the vicinity of the 1- and 2-positions of the substrate; it is conjectured that the metal-ion-activated water molecule (hydroxy anion) acts as an acid-base catalyst and that isomerization takes place as the hydrogen anion moves between the 1- and 2-positions.
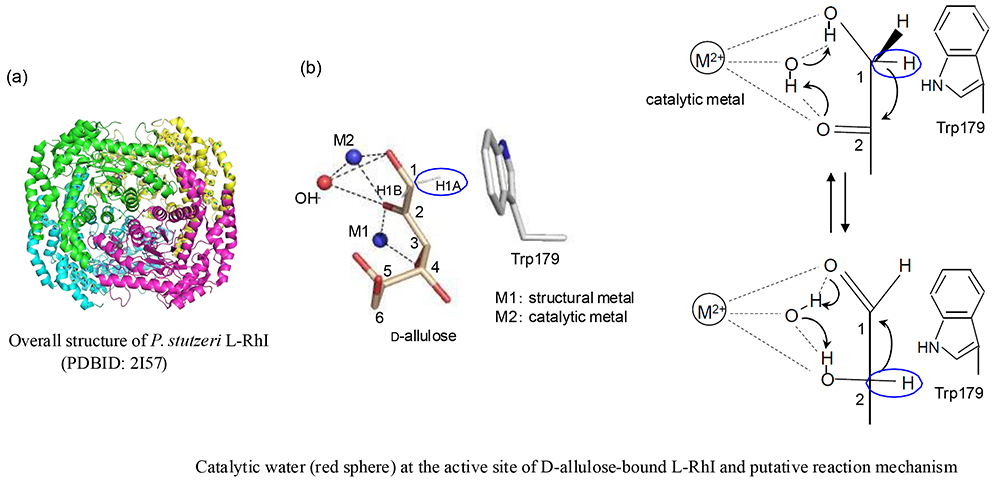
In the catalytic reaction of PsL-RhI, broad substrate specificity has been identified, and its association with D-xylose isomerase has been reported from the viewpoint of substrate specificity34. While the overall structures of PsL-RhI and EcL-RhI are very similar to each other, both have a flexible loop region involved in the formation of active sites (Fig. 6). The loop region in monomeric structure shown in red shows a marked difference at the dimer unit interface. The loop region of EcL-RhI is the active site within its subunit structure, whereas that of PsL-RhI is part of the active site located in the adjoining subunit. Because the active site of D-xylose isomerase is also known to have such a nested structure, the substrate characteristics and structural features of PsL-RhI were confirmed to have an association with those of D-xylose isomerase33.
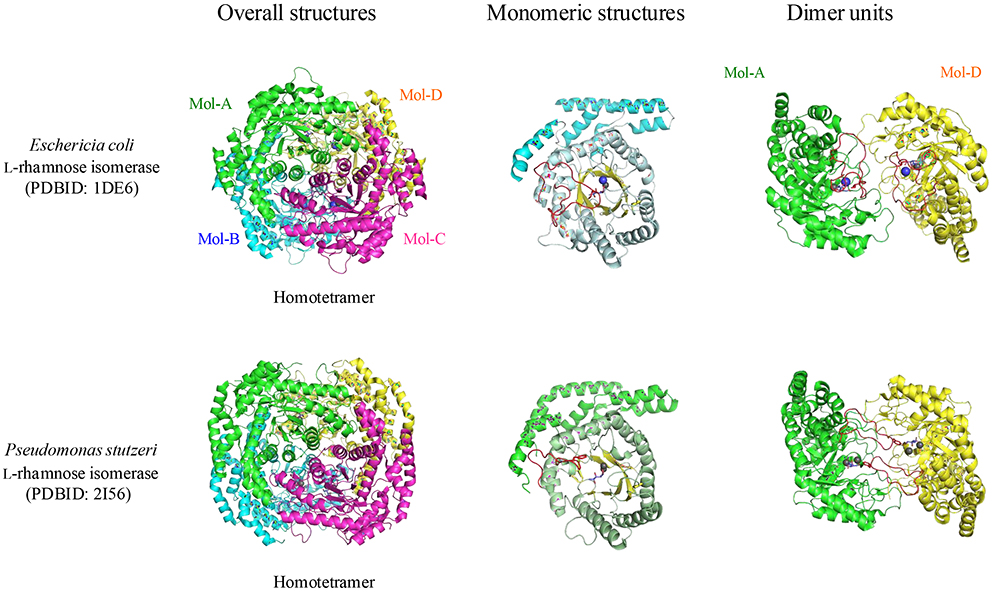
Although the structures of L-RhI35 from Bacillus halodurans and L-RhI36 from Lactobacillus rhamnosus have been reported, the corresponding loop region of L-RhI from B. halodurans remains invisible due to its fluctuations, and L-RhI from L. rhamnosus forms the same type of dimer unit as EcL-RhI. Recently, phylogenetic analysis showed that the L-RhI, PsL-RhI, is more closely related to D-xylose isomerase than EcL-RhI37, again demonstrating the broad substrate recognition by PsL-RhI as a rare sugar production enzyme.
In this fourth article of the Rare Sugars series, we have described the structures and catalytic reaction mechanisms of ketose 3-epimerase and L-rhamnose isomerase as the basic enzymes catalyzing rare sugar production. These results represent our attempt to obtain key information on rare sugar production, marking an important milestone in the mass production of rare sugars. The fifth article of the series will describe the status of the industry-academia-government collaboration to develop new applications of rare sugars in Japan. As new rare sugar functionalities have been found to enable the development of not only novel food applications but also advanced industrial applications worldwide, the need for diverse rare sugars has been increasing and has stimulated aggressive competition in research on enzymes and efficient methods of rare sugars production. In this situation, many research groups have been reporting the discovery of enzymes of broad substrate specificity capable of catalyzing the production of a wide variety of rare sugars, highly productive, efficient, and more environmentally resistant enzymes, and the creation of genetically modified enzymes with site-directed amino acid mutations based on findings of their structures. The search for microorganisms containing enzymes capable of producing new rare sugars is ongoing. We hope that the acquisition of these new enzymes and the microorganisms that contain them will lead to the mass-production of a wide variety of rare sugars, advance applied research on rare sugars to enable the discovery and understanding of new functionalities of rare sugars, and successful application of this technology to our society at large.