
Kensaku Fukunaga
Assistant Professor, Department of Endocrinology and Metabolism, Faculty of Medicine, Kagawa University
Dr. Fukunaga graduated from the Faculty of Medicine at Kagawa University in 2009 and completed his four-year doctoral course in the Graduate School of Medical Sciences, Kagawa University, Kagawa University in 2018. He specializes in endocrinology and metabolism, engaging in both clinical practice and research.
His research focuses on the clinical application of rare sugars in relation to adipose tissue, obesity, and glucose metabolism; novel treatment strategies for patients with type 2 diabetes complicated by metabolic dysfunction-associated steatotic liver disease (MASLD); identification and evaluation of predictive factors for lifestyle diseases using health checkup data; and implementation of problem-solving experiential learning programs (“Future Classroom”) aimed at preventing lifestyle diseases in children. All of these efforts are directed toward clinical research with a view to social implementation.
Board Certified Member of the Japanese Society of Internal Medicine (JSIM), Fellow of the JSIM (FJSIM), JSIM appointed physician for fellowship training, Diabetologist and Consultant Diabetologist of the Japan Diabetes Society, Board Certified Endocrinologist and Certified Endocrine Educator by the Japan Endocrine Society, Occupational Physician of the Japan Medical Association.
Diabetes and obesity-related metabolic disorders pose a significant global health burden, highlighting the need for innovative therapeutic strategies. The cornerstone of managing these conditions lies in lifestyle modifications, including diet and physical activity.
Excessive sugar intake, driven by modern dietary habits, has been implicated in the rising prevalence of diabetes, obesity, and cardiovascular diseases1. This has spurred interest in safer sugar alternatives. While artificial sweeteners have been explored for their potential benefits in managing obesity and diabetes2, some studies suggest they may adversely affect glucose metabolism3, necessitating careful evaluation of their use.
D-allulose, a naturally occurring rare sugar, has gained attention as a promising alternative. Our clinical research suggests that D-allulose may improve glucose metabolism in patients with type 2 diabetes. This review consolidates the current evidence on the effects of D-allulose on diabetes, obesity, and fat metabolism, and assesses its potential for clinical application.
Rare sugars are monosaccharides and their derivatives that naturally occur in limited quantities. Research has identified diverse biological activities associated with these sugars, suggesting potential health benefits.
D-Allulose is a rare sugar with a low caloric value (0.4 kcal/g). Although absorbed into the bloodstream, most of it is excreted in the urine, minimizing its contribution as an energy source4. While excessive intake may cause mild gastrointestinal symptoms, the maximum no-observed-adverse-effect level (NOAEL) is 0.55 g/kg body weight per day5, indicating that D-allulose is generally safe when consumed within recommended limits.
D-Allulose lowers postprandial glucose levels through several pathways. In the small intestine, it inhibits α-glucosidase activity, thereby reducing glucose and fructose absorption6. In the liver, it enhances glucokinase expression, promoting glycogen synthesis while suppressing gluconeogenesis and hepatic glucose release, leading to better glycemic control7.
Additionally, D-allulose may improve insulin sensitivity and exert protective effects on pancreatic β-cells8. These mechanisms collectively suggest a multifaceted role in glucose regulation.
D-Allulose may aid in weight management by modulating appetite and energy metabolism. It has been shown to enhance glucagon-like peptide-1 (GLP-1) secretion, which suppresses appetite via vagal afferent signaling to the hypothalamus9.
Moreover, emerging evidence suggests that D-allulose promotes the conversion of white adipose tissue into beige adipose tissue, a process associated with increased thermogenesis and energy expenditure. This beiging effect is linked to upregulation of uncoupling protein-1 (UCP-1), a key regulator of mitochondrial heat production. These findings indicate that D-allulose may contribute to fat reduction through multiple metabolic pathways, warranting further investigation.
Human studies have demonstrated that D-allulose lowers postprandial glucose levels in both healthy individuals and those with impaired glucose tolerance. In healthy subjects, D-allulose reduces postprandial glucose in a dose-dependent manner while also modulating insulin secretion10-12.
In individuals with impaired glucose tolerance (prediabetes), consuming 5 g of D-allulose alongside a test meal (425 kcal: 84.5 g carbohydrates, 13.3 g protein, 3.7 g fat) significantly reduced postprandial blood glucose levels13. These findings highlight D-allulose as a potential dietary intervention for improving glucose regulation.
To assess its clinical utility, we examined whether D-allulose could be integrated into dietary therapy for diabetes14. Our study compared a conventional diabetic diet—where caloric intake was calculated based on body weight and physical activity—with a modified diet incorporating 8.5 g of D-allulose per meal, maintaining identical macronutrient and caloric composition.
The D-allulose-enriched diet demonstrated superior postprandial glucose suppression in patients with type 2 diabetes (Figure 1). This study, the first to use continuous glucose monitoring (CGM) to evaluate D-allulose in dietary therapy, supports its potential as an innovative nutritional strategy for diabetes management.
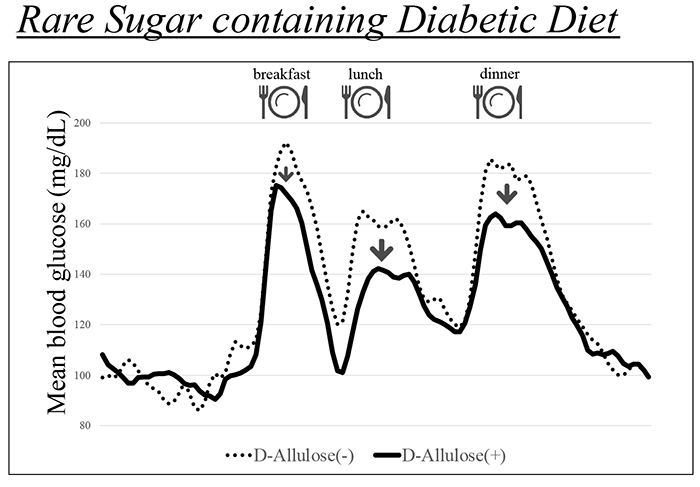
D-Allulose has been granted Generally Recognized as Safe (GRAS) status by the U.S. Food and Drug Administration (FDA), highlighting its safety as a food ingredient. The global prevalence of diabetes is rising, with Asia experiencing particularly rapid growth.
In Malaysia, for example, diabetes rates are steadily increasing. A significant challenge arises during Ramadan, when Muslim patients fast from dawn to sunset (approximately 13 hours). This fasting period poses risks of daytime hypoglycemia, while Iftar, the post-sunset meal, often leads to excessive food intake and heightened risks of postprandial and nocturnal hyperglycemia.
To address this issue, we conducted a study in patients with type 2 diabetes during Ramadan, investigating the effects of D-allulose on glycemic fluctuations using CGM15. The results showed a significant reduction in postprandial glucose peaks and the incremental area under the glucose curve (iAUC) after Iftar. These findings suggest that D-allulose could help improve glycemic control during Ramadan (Figure 2). This research highlights the potential of D-allulose as a novel dietary intervention for diabetes management, particularly in culturally specific settings where fasting-induced glycemic fluctuations pose significant health risks.
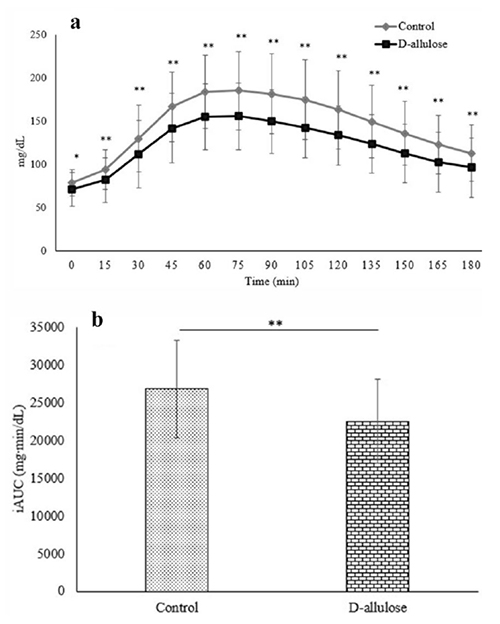
D-Allulose represents a promising dietary strategy for diabetes and obesity management, given its ability to lower postprandial glucose levels, enhance GLP-1 secretion, and promote fat burning. As a naturally derived, low-calorie sugar with potential metabolic benefits, it aligns with modern consumer preferences for healthier alternatives.
Further clinical research is essential to validate its therapeutic efficacy. Rigorous trials assessing its long-term metabolic effects will be crucial for advancing its medical applications and integrating D-allulose into evidence-based dietary strategies for diabetes and obesity treatment.