
Hisatoshi Hanamatsu
Specially Appointed Lecture
Institute for Glyco-core Research, Nagoya University, Tokai National Higher Education and Research System
He received his PhD in life sciences from Hokkaido University in 2014. He did postdoctoral training at Hokkaido University from 2014 in Graduate School of Advanced Life Science and Frontier Research Center for Post-Genome Science and Technology, Hokkaido University. He did postdoctoral training at Hokkaido University from 2017 in Department of Advanced Clinical Glycobiology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University. In 2020, he was a Specially Appointed Assistant Professor in Faculty of Medicine and Graduate School of Medicine, Hokkaido University. Form 2023, he is a Specially Appointed Lecture in Institute for Glyco-core Research, Nagoya University. His research interests focus on the development of sialic acid linkage-specific derivatization and total glycome analysis.

Jun-ichi Furukawa
Designated Professor
Institute for Glyco-core Research, Nagoya University, Tokai National Higher Education and Research System
Visiting Professor
Department of Orthopaedic Surgery, Faculty of Medicine and Graduate School of Medicine, Hokkaido University
Jun-ichi Furukawa graduated from Faculty of Science at Hokkaido University in 1996, received his Ph.D. in Environmental Earth Science at Hokkaido University in 2001. Then, he worked as a JSPS postdoctoral, a postdoctoral fellow (2002–2006), and as a research assistant professor (2006–2016) in Faculty of Science, Hokkaido University. In 2016, he was a Specially Appointed Associate Professor of Faculty of Medicine, Hokkaido University. He has been in his current position since 2022. His current research interest include bioorganic chemistry and analytical chemistry focused on glycans. He is studying the comprehensive glycomic analysis using his original analytical tools.
In segment 2 of the Human Glycome Atlas Project (HGA), we are aiming for the comprehensive analysis of N-, O-, and glycosphingolipid (GSL)-glycans, glycosaminoglycans (GAG), and free oligosaccharides, the generation of a human glycome catalog (total human plasma glycome), and the construction of resulting knowledgebase called TOHSA. In this section, we introduce the glycomic techniques used to elucidate the GSL-glycome in human serum and plasma.
Various types of glycans cover all eukaryotic cell surfaces and play important roles in numerous biological events. Therefore, glycans are important as the third life chain following nucleic acids (genes) and proteins. Glycans are classified according to the types of glycoconjugate. Glycans at Asn or Ser/Thr residues in proteins are designated N-glycans or O-glycans, respectively. Most glycosaminoglycans are attached to core proteins (called proteoglycans) and hyaluronic acid without sulfate groups is present as free glycosaminoglycan. Glycans linked to hydrophobic ceramides are glycosphingolipids (GSLs), which are the predominant glycolipids in humans. We have reported that comprehensive glycomic methods, called total glycomics, can be used with glycan preparation methods and analytical techniques such as matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectroscopy (MS) and high performance liquid chromatography to measure different classes of glycans1-5. In addition, we developed several sialic acid linkage-specific alkylamidation (SALSA) procedures toward N-, O-, and GSL-glycans containing sialic acid residues at the non-reducing end. The analytical methods of N- and O-glycans via lactone ring-opening aminolysis (aminolysis-SALSA) have already been reported in this series (Glycoforum. 2024 Vol.27 (3), A9, Glycoforum. 2024 Vol.27 (6), A22). We further reported a unique method of distinguishing sialylated glycan isomers via sialic acid linkage-specific conversion from ester to amide by MS analysis6. Herein, we want to introduce 1) the analytical method of GSL-glycans and the sialic acid linkage-specific derivatization by lactone-driven ester-to-amide conversion (LEAD-SALSA) and 2) the development of an automated system to prepare human serum or plasma GSL-glycans intended for use in a large-scale cohort study.
Glycosphingolipids (GSLs) compose of ceramide moieties consisting of a fatty acid linked to backbone sphingosine and carbohydrate moieties such as glucose, galactose, and a variety of glycans. GSLs are unique amphipathic molecules containing both hydrophilic and hydrophobic regions, and are present in the plasma membrane lipid raft, which is a cluster of sphingomyelins, cholesterols, and specific proteins. GSLs affect membrane physical properties and provide specific recognition sites for various biological events. These bioactive GSL molecules consequently affect the pathophysiology and pathogenesis of various diseases, including various GSL lysosomal storage diseases, various types of cancer, infections, atherosclerosis, diabetes, and central nervous system diseases7-11. Serum GSLs containing gangliosides are primarily associated with apoproteins, resulting in the formation of various lipoproteins and circulation in the blood12. GSLs with over 300 kinds of glycan structures have been reported in LIPID MAPS(https://www.lipidmaps.org). Ceramides also have a diversity of structures due to different length of fatty acids, and different contents of double bonds and hydroxyl groups. For these reasons, analysis of GSLs containing both complexities is most challenging. Our strategy in GSL-glycomics is to release glycan moieties by endoglycoceramidase (EGCase) digestion for the exclusion of ceramide moieties with complex diversity. The method of GSL-glycan analysis is introduced in the next section.
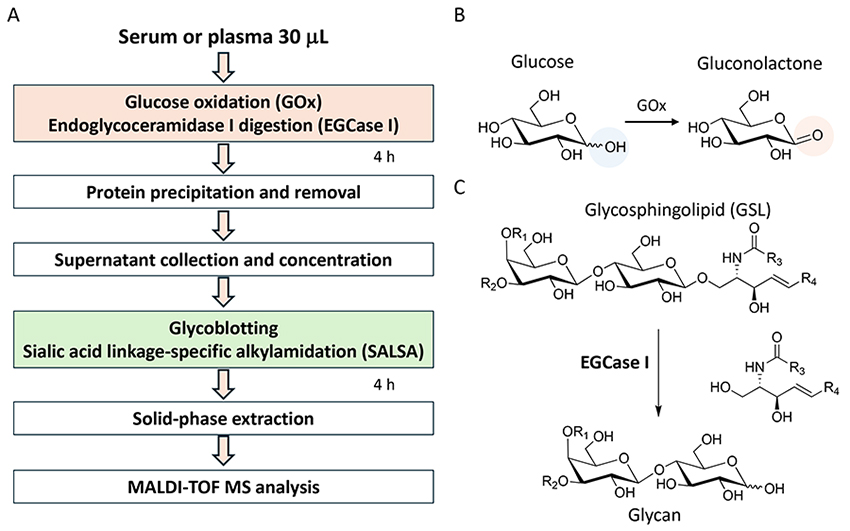
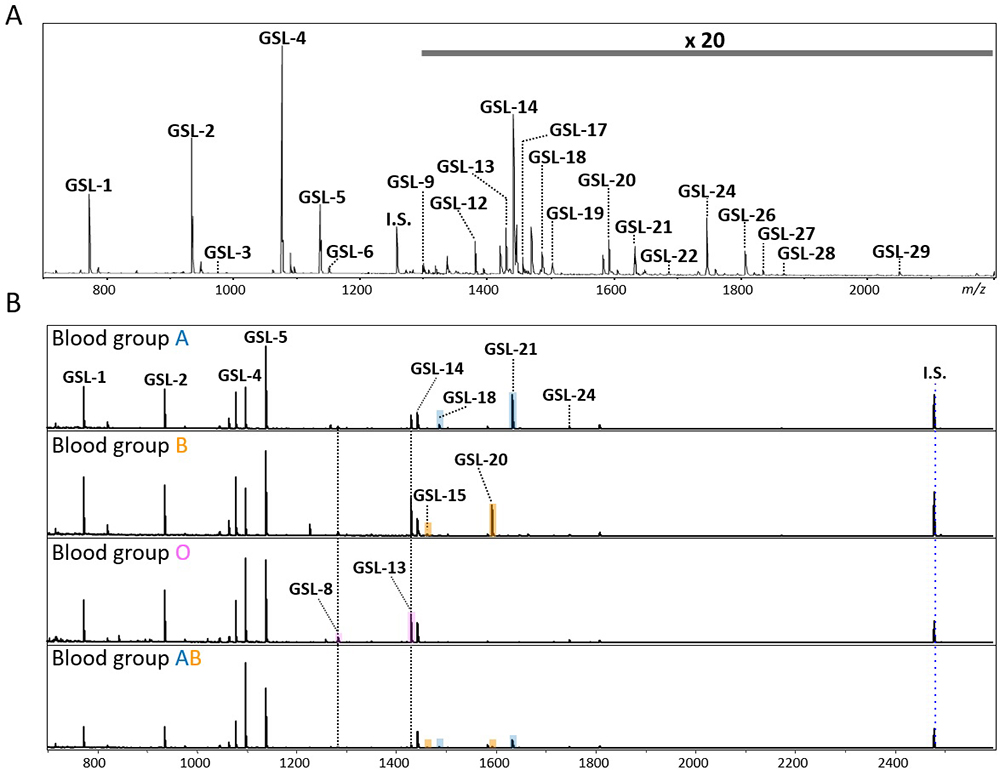
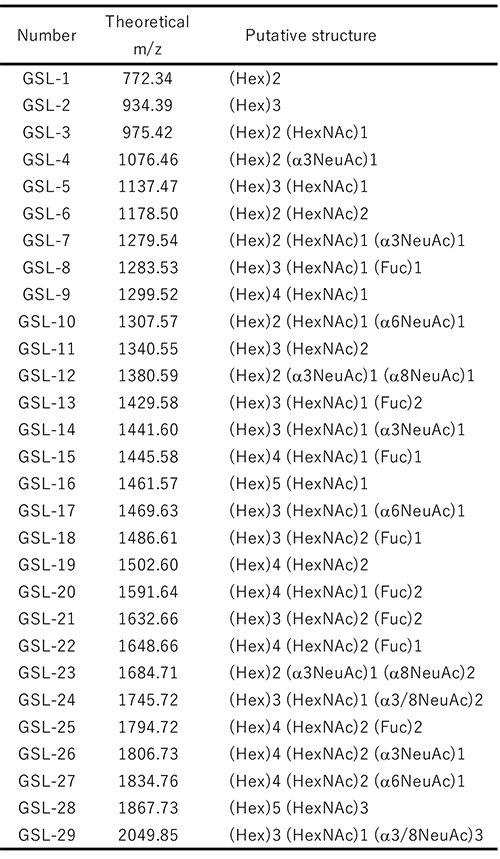
A streamlined protocol of GSL-glycan analysis is shown in Figure 1A. A 30 µL serum or plasma is used for GSL-glycomic analysis, which is larger than the volume used for N- and O-glycan analyses since the abundance of GSL-glycans is extremely low and about 1/100 that of N-glycans on proteins. In addition, the normal glucose concentration in human blood is reportedly about 4.4–6.1 mmol/L13, and the glucose with aldehyde group is chemoselectively captured as well as GSL-glycans by BlotGlyco beads. Therefore, a high concentration of glucose interferes with the measurement of GSL-glycans in serum. In our protocol, we use a glucose oxidase (GOx) before glycan capturing. GOx oxidizes glucose to gluconolactone without an aldehyde group (Figure 1B). Next, GSL-glycans are released from ceramides by endoglycoceramidase I (EGCase I) digestion (Figure 1C). Previously, we reported that the employment of both EGCase I and II facilitated the analysis of cellular GSL-glycans4; however, excess EGCase I alone is enough to release GSL-glycans with broad specificity from human serum GSLs14. After simultaneous digestion by GOx and EGCase I, a large amount of glycoproteins including N- and O-glycans must be removed from reaction mixtures containing extremely low GSL concentrations before GSL-glycomics can be performed. Instead of fractionation by centrifugation, protein removal plate with 96 well format has been used for automation. A digestion mixture containing GSL-glycans is added to a 9-fold volume of methanol in a 96-well protein removal plate and the passthrough fraction containing released GSL-glycans is recovered. Released GSL-glycans are purified by glycoblotting procedure and captured GSL-glycans can also be derivatized on the solid-phase. After sialic acid linkage-specific alkylamidation (SALSA), GSL-glycans are released from beads and labeled with Nα-([aminooxy]acetyl) tryptophanylarginine methyl ester (aoWR) by the imine exchange reaction15. Finally, GSL-glycans are measured by MALDI-TOF MS after removal of excess aoWR. The MALDI-TOF MS spectrum of the GSL-glycan derived from human serum using the above method is shown in Figure 2A. The 29 detected GSL-glycans are listed in Table 1. We demonstrated that blood type-specific GSL-glycans are predominantly present in serum and plasma16. As shown in Figure 2B, mono- (GSL-8: [Hex]3[HexNAc]1[Fuc]1) and di-fucosylated GSL-glycans (GSL-13: [Hex]3[HexNAc]1[Fuc]2) were observed in all blood types. In blood type A serum, GSL-18: (Hex)3(HexNAc)2(Fuc)1 and GSL-22: (Hex)3(HexNAc)2(Fuc)2, which are presumed to have terminal α-GalNAc, were observed. In blood type B, GSL-15: (Hex)4 (HexNAc)1(Fuc)1 and GSL-20: (Hex)4(HexNAc)1(Fuc)2 containing Gal instead of GalNAc were detected as blood type specific antigens. In blood type AB serum, both blood type A and B glyco-antigens were detected. However, these antigens were not detected in a non-secretor (Lea+,Leb−) lacking fucosyltransferase 2 gene (FUT2, also known as Se). In the analytical method shown as Figure 1A, not only GSL-glycans but also fructo-oligosaccharides (fOSs) were simultaneously detected. Interestingly, similar to GSL-glycans, blood type-specific glycans were also present among the fOSs. For details, please consult our previous paper16.
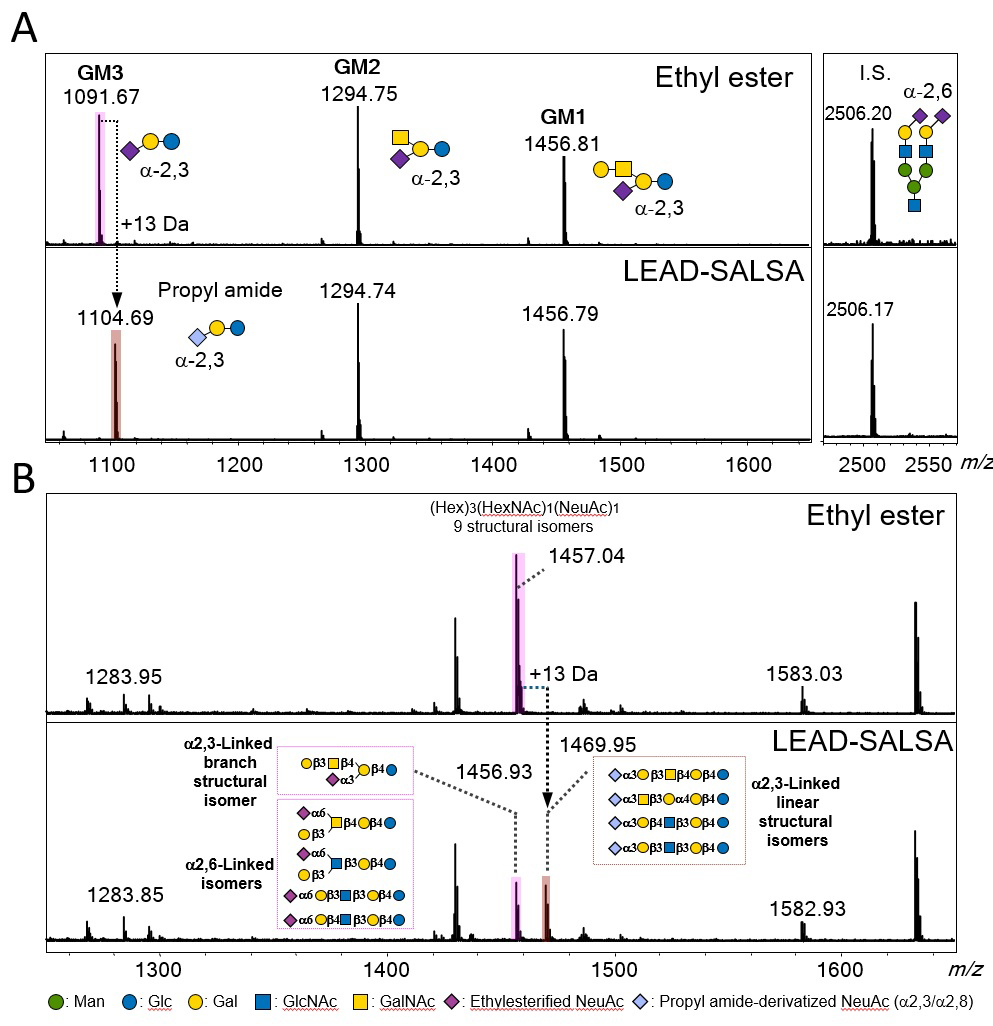
Previously, we demonstrated several sialic acid linkage-specific derivatization methods based on intramolecular lactone formation for distinguishing sialyl linkages by MS analysis6,17-19. We introduce one of them, the LEAD-SALSA method6. Esterified sialyloligosaccharides were observed to be more stable than non-modified sialyloligosaccharides by MALDI-TOF MS analysis; however, differences of sialyl linkage isomer could not be observed by MS analysis. Recently, we revealed that methyl esterified α2,3-sialylated glycans were selectively converted to amides only by mixing with amine reagents, whereas α2,6-sialylated glycans remained as esters. Furthermore, we found that the stability of lactone was important for selective ester-to-amide conversion based on experiments and density functional theory calculations of reaction energies for lactone formation. By using energy differences of lactone formation, the LEAD-SALSA method can be used not only for identifying the sialyl linkage but also for distinguishing the branching structure of galactose linked to sialic acid. We used the LEAD-SALSA method to perform GSL-glycomics of bovine GM1, GM2, and GM3 (Figure 3A). Ethyl esterified GM3 glycan was converted to the corresponding amide product. The GM1 and GM2 glycans containing the branching structure of Gal remained in their ethyl esterified forms. Next, we performed the analysis of human serum GSL-glycans (Figure 3B). In LEAD-SALSA method, the signal at m/z 1456 was converted to a signal of similar intensity at m/z 1469 (its propyl amide forms). These results indicated that 50% of linear glycans containing α2,3-sialic acid were present in this human serum.
In the HGA, the development of automated sample preparation platforms is essential for large-scale glycomic analysis of various glycoconjugate-glycans. We have previously constructed automated N- and O-glycan preparation systems (Glycoforum. 2024 Vol.27 (3), A9, Glycoforum. 2024 Vol.27 (6), A22). In this section, we introduce the automated GSL-glycan preparation system that uses the protocol described above (Figure 4A). This system is compatible with various 96-well format plates and uses an 8-channel multi-syringe to dispense volumes ranging from 1 to 2500 µL. The thermostatic oven can maintain temperature up to 100°C for enzymatic reaction and glycan capture. Vigorous mixing on a plate shaker unit promotes the SALSA reaction. In addition, purified and derivatized GSL-glycans in serum or plasma are mixed with matrix and spotted on a MALDI plate for MS measurement. Although this system is almost identical to the N-glycan system, it is newly equipped with a positive pressure unit (Figure 4B, red line unit). The unit enables automated protein removal from serum samples using protein removal filter plates instead of ethanol precipitation with centrifugation. This system allows all processes for GSL-glycan preparation from 96 serum or plasma samples to be completed within 16 h.
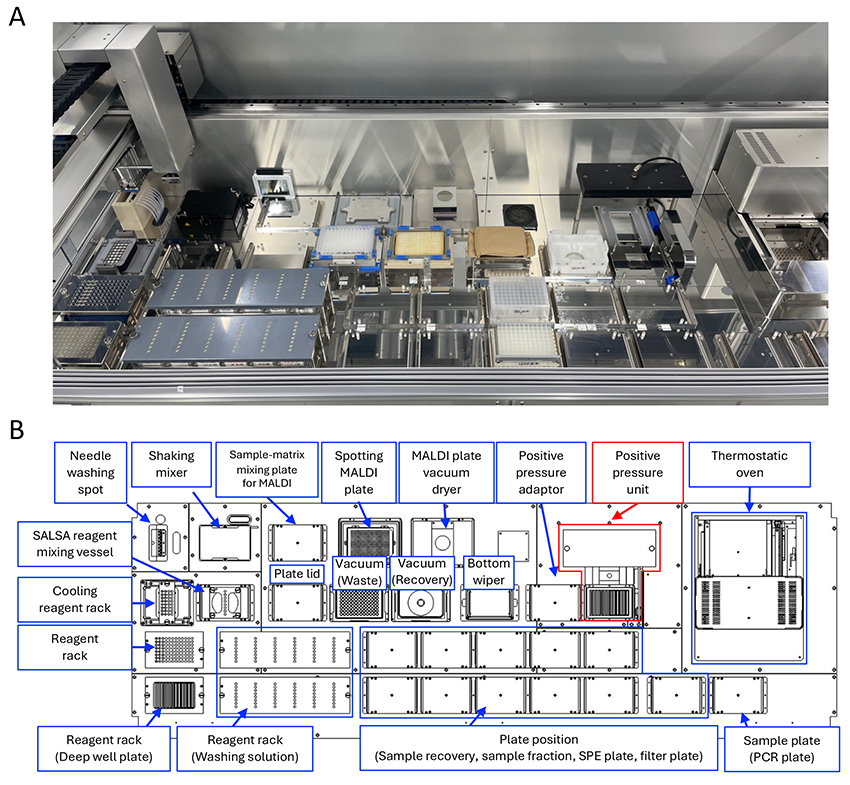
We have developed an automated sample preparation system for use in large-scale GSL-glycan analysis. We also demonstrated that various GSL-glycans containing blood type-specific glyco-epitopes could be detected in human serum and plasma. Moreover, we are ready to automate the preparation and measurement of serum and plasma N- and O-glycans. We will need to develop software to quickly and accurately analyze the enormous volume of these glycan data. Future studies should attempt to elucidate the functions of serum and plasma glycans in biological events, and discover new biomarkers.