
Satoshi Fujita
Professor, Graduate School of Engineering, University of Fukui
He graduated from the Faculty of Engineering at Kyoto University in 1997, completed a master’s degree in Polymer Chemistry, and earned a Ph.D. in Engineering at Kyoto University in 2009. He has worked in research and development at a brewery, a biotechnology firm, and a clinical testing company. He is also a certified Professional Engineer Japan in Biotechnology and Bioengineering. While at a clinical testing company, he was seconded to the Institute for Frontier Medical Sciences of Kyoto University, where he focused on research into cellular microenvironments to control cell behavior through material design. He joined the University of Fukui in 2011 and has held his current position there since 2022. His expertise includes electrospun nanofibers and biopolymer hydrogels. He serves as Secretary of the Biotechnology Division of The Institution of Professional Engineers, Japan, Chairman of the Medical Materials Research Committee of The Society of Fiber Science and Technology, and a Councilor of the Japanese Society for Biomaterials.
Characterized as fibrous materials with diameters ranging from submicrons to nanometers, electrospun nonwoven nanofibers are promising candidates for use as culture substrates and in regenerative medicine. Their distinct properties, including high specific surface area, porosity, and light weight, make them particularly suitable for these applications. Nanofibers can be fabricated from a variety of polymers, frequently exhibiting performance superior to that of conventional materials. This review article focuses on electrospinning technologies for producing nanofibers from polysaccharides and their derivatives.
Methods for fabricating nanofibers include melt-blowing, extrusion, phase separation, and electrospinning. Among these, electrospinning by high-voltage whipping is widely used for producing nanofiber nonwoven fabrics from polymer solutions or polymer melts. This method is advantageous due to its simplicity and cost-effectiveness. Adjustment of electrospinning parameters including polymer type, solution concentration, solvent composition, applied voltage, injection speed, and environmental humidity allows precise control over fiber diameter and morphology, such as beads, mesh, mats, and monofilaments. As a result, it enables the design of materials tailored for a wide range of applications.
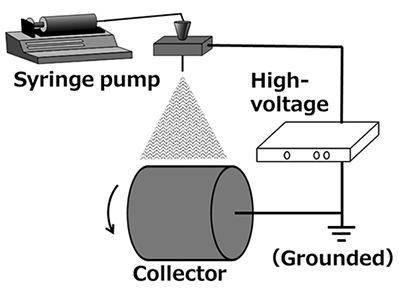
Electrospinning methods are broadly classified into two types: melt-based and solution-based. The melt-based method uses polymers melted at high temperatures, whereas the solution-based method employs polymers dissolved in solvents. In both approaches, polymers are ejected from a nozzle to a grounded collector under the influence of a high-voltage electric field, where they are stretched to form fine fibers (Figure 1). The melt-based method can accommodate specialized polymers, such as engineering plastics that are difficult to dissolve in solvents. However, because it requires high temperatures to melt the polymers, it is unsuitable for heat-sensitive polymers and natural biopolymers prone to thermal degradation. In contrast, the solution-based method is applicable to a wider variety of polymers. In this process, as the solvent evaporates from the polymer solution droplets ejected from the nozzle, the charge becomes concentrated, and repulsive forces between the charges lead to the formation of fine fibers. All examples presented in this article involve the fabrication of nanofibers from polysaccharides using the solution-based electrospinning method. From this point onward, references to electrospinning will specifically refer to the solution-based technique.
While electrospinning can be applied to almost any polymer given a suitable solvent, some polymers tend to form nanofibers more than others. Generally, highly polar or crystalline polymers are easier to spin into fibers, whereas amorphous polymers tend to be more difficult. Polylactic acid and poly-ε-caprolactone, both known as biodegradable materials, are representative examples of polymers that are easily electrospun into nanofibers. Polyurethane also exhibits good spinnability, resulting in stretchable nanofiber sheets. Additionally, water-soluble polymers such as polyethylene oxide (PEO), polyvinyl alcohol (PVA), and polyvinylpyrrolidone (PVP) are known to be readily processed into nanofibers. In contrast, biopolymers such as collagen, alginate, and hyaluronic acid (HA) tend to gel in concentrated aqueous solutions, making them difficult to spin. This article focuses on the electrospinning of polysaccharides, a group of biopolymers that are challenging to electrospin, and introduces processes developed to achieve successful nanofiber formation.
Alginate, a polysaccharide derived from seaweed, consists of a random copolymer of α-L-guluronic acid and β-D-mannuronic acid. In the presence of metal ions, such as calcium, alginate forms a cross-linked structure known as the "egg-box structure," resulting in a hydrogel with high water absorbency and biocompatibility. These properties make alginate a promising material for applications as food additives, in drug delivery systems for protein- and peptide-based therapeutics, and in tissue engineering. Additionally, chemical modification of the carboxyl groups on the alginate side chains has been explored to create smart materials that respond to stimuli such as light, temperature, or ultrasound, as well as to develop cell-adhesive materials through the incorporation of cell-adhesion molecules.
Conventionally, alginate hydrogels have been used as isotropic bulk materials. However, electrospinning enables the fabrication of anisotropic materials based on fiber alignment. Despite this potential, electrospinning of alginate is challenging due to its rigid chain structure, which results in insufficient chain entanglement1. To address this, blending with water-soluble polymers or hydrogen-bonding compounds, such as PEO, PVA, or glycerol, has been employed to facilitate nanofiber production via electrospinning2-4.
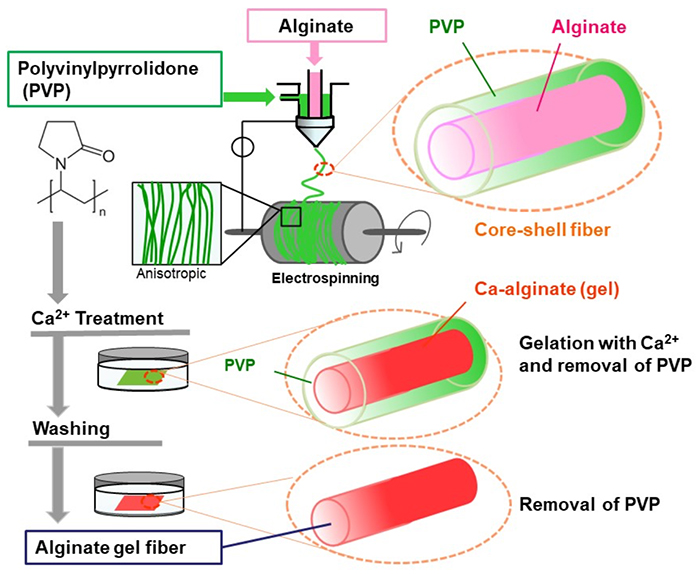
We focused on core-shell electrospinning to produce nanofibers composed solely of alginate, without the need for additives. This method uses alginate as the core material and PVP as the shell, which aids in fiber formation. The resulting core-shell fibers encapsulate the alginate solution within the PVP shell5. PVP, known for its good spinnability, serves as a guide during fiber formation. When the fibers are immersed in a calcium ion-containing solution, the encapsulated alginate cross-links and gels, while the outer PVP dissolves and is removed. This process results in the formation of water-insoluble alginate hydrogel fibers (Figure 2). The obtained product retains fiber alignment and inter-fiber porosity, enabling the fabrication of various three-dimensional structures, such as vascular tubes. Notably, this method simplifies the creation of nanofiber structures that mimic the extracellular matrix (ECM). Additionally, the use of chelating agents, such as ethylenediaminetetraacetic acid, facilitates the dissolution of the gel, allowing for the easy recovery of fiber-adherent cells after proliferation.
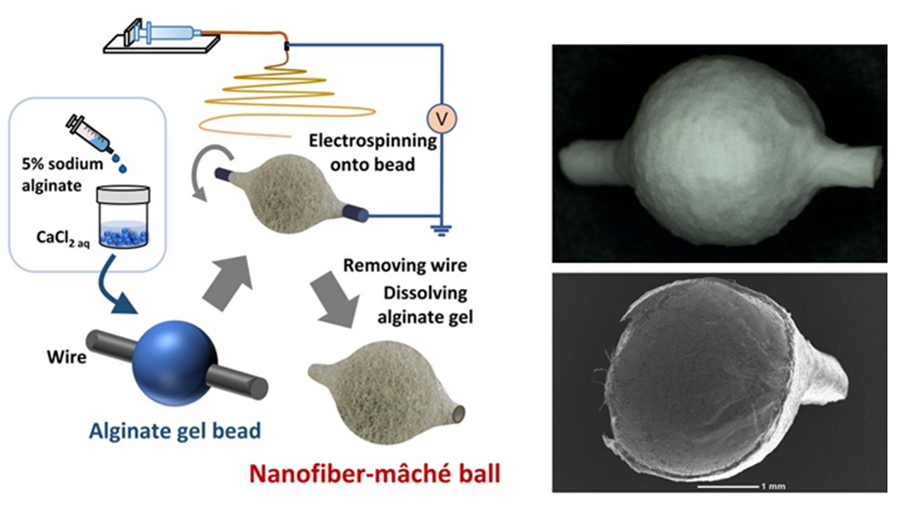
The gelation and dissolution properties of alginate can be further utilized in the fabrication of three-dimensional structures. We developed an electrospinning method that employs alginate hydrogel as a mold (Figure 3)6. In this approach, alginate hydrogel beads are directly used as the collector. Since alginate gel, cross-linked in the presence of calcium ions, is conductive, a grounded metal wire is passed through the gel bead, which is rotated while nanofibers are deposited onto its surface. This process produces a nanofiber-coated three-dimensional structure on the surface of the bead. Subsequently, the gel is dissolved using a chelating agent, leaving behind a three-dimensional nanofiber structure. This technique enables the production of hollow structures, similar to papier-mâché dolls. Moreover, by adjusting the rotation speed of the collector, the alignment of fibers on both the inner and outer surfaces, as well as the surface microstructure, can be precisely controlled. A key advantage of this technique is the flexibility in fabricating the alginate hydrogel mold itself, allowing hydrogels of any size and shape to be created using a 3D printer7. This method holds significant potential for applications in regenerative medicine and drug discovery, as it facilitates the creation of three-dimensional scaffolds that mimic the complex structures of organs and tissues.
HA is a naturally occurring polysaccharide composed of N-acetyl-D-glucosamine and D-glucuronic acid, commonly found in animal tissues. HA is utilized in various fields, including cancer therapy and cosmetics, and is particularly notable for its role as a key component of the ECM. Its use in hydrogels and films, such as wound dressings and cell culture scaffolds, has garnered significant attention. For instance, HA hydrogels formed via intermolecular cross-linking through the oxidation of thiol-modified HA derivatives8, and HA films combined with materials like chitosan and collagen9, have been reported.
Nanofiber fabrication from HA increases the surface area compared to conventional gels and films, introducing anisotropy and porosity. This enhances its potential for delivering bioactive molecules, growth factors, and drugs, expanding its applications to tissue engineering, wound healing, and drug delivery systems. One of the earliest reports on HA nanofiber fabrication involved the electrospinning of thiol-modified HA derivatives by Ji et al.10. In this study, a solution containing PEO and polyethylene glycol diacrylate was electrospun, followed by cross-linking through disulfide bond formation and conjugate addition reactions between thiol and acrylate groups. The resulting nanofibers functioned as scaffolds for cell culture.
However, concerns regarding the safety of chemically modified HA, particularly in medical and cosmetic applications, persist. This has led to further research into the electrospinning of unmodified HA. A subsequent study revealed that highly polar organic solvents, such as dimethylformamide (DMF), can be used to electrospin HA11. Nonetheless, safety concerns about the use of organic solvents remain, increasing the demand for electrospinning techniques that utilize aqueous solutions for unmodified HA.
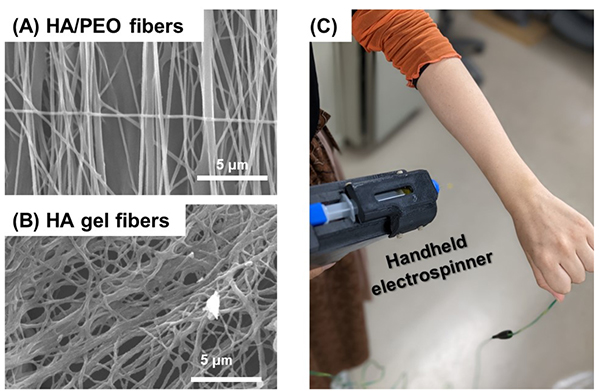
We attempted to enhance the spinnability of unmodified HA by incorporating PEO into an aqueous HA solution for electrospinning in an aqueous system12 (Figure 4). As mentioned above, PEO has been used to improve the spinnability of materials that are otherwise challenging to electrospin; however, its application to unmodified HA had not been previously reported. In this study, the pH was adjusted to reduce intramolecular charge repulsion, thereby improving spinnability. After electrospinning, a combination of heat treatment and quenching was employed to remove PEO, successfully yielding HA hydrogel fibers that remained insoluble and stable in water for prolonged periods. Additionally, the potential for cosmetic and wound dressing applications was demonstrated by using handheld electrospinning technology to directly spin HA/PEO fibers onto the human body (Figure 4C).
Chitin is a polysaccharide composed of N-acetylglucosamine units linked by β-1,4 bonds and is primarily found in shrimp and crab shells, insect exoskeletons, and fungal cell walls in mushrooms, where it provides hardness and strength. Due to its insolubility in water and chemical stability, chitin is challenging to utilize. In contrast, chitosan, derived from the deacetylation of chitin, is highly water-soluble and easier to handle, making it widely used in fields such as medicine, food processing, cosmetics, and agriculture.
Research on chitosan nanofibers has advanced significantly, and due to their biocompatibility, antimicrobial properties, and porous structure, they hold promise for various applications. Notably, chitosan is gaining attention as a wound dressing material due to its antimicrobial activity, hemostatic properties, and wound healing promotion effects.
Chitosan can be dissolved in acidic solutions, such as acetic acid, to create solutions suitable for electrospinning13. As with other polysaccharides, the spinnability of chitosan can be improved by adding PEO14 or PVA15. Other solvents, such as trifluoroacetic acid, have also been reported16. However, the resulting chitosan nanofibers are water-soluble and mechanically weak, necessitating the development of various cross-linking methods to improve their stability and mechanical properties. For instance, chemical cross-linking using glutaraldehyde16 or genipin13, as well as the formation of polyelectrolyte complexes through electrostatic interactions between positively charged chitosan and negatively charged sodium alginate17, have been reported.
Since chitin is insoluble in water, electrospinning it is challenging. However, it has been demonstrated that by dispersing chitin in a chitosan solution, chitin-chitosan composite nanofibers can be successfully spun18,19.
Due to the limited availability of solvents capable of dissolving cellulose, it is generally considered poorly suited for electrospinning. Ionic liquid, one of the few known solvents for cellulose, has been employed in electrospinning. Xu and colleagues successfully dissolved natural cellulose using the ionic liquid 1-allyl-3-methylimidazolium chloride (AMIMCl) and produced ultrafine fibers via electrospinning20. Although AMIMCl can dissolve cellulose at room temperature, its high viscosity presents challenges. To address this, dimethyl sulfoxide (DMSO) was added as a co-solvent to reduce both viscosity and surface tension, enabling continuous spinning. By optimizing factors such as cellulose concentration, the AMIMCl/DMSO ratio, and environmental humidity, smooth and uniform fibers were obtained. Subsequent studies have also reported the use of various imidazolium-based ionic liquids for cellulose electrospinning21. However, residual ionic liquids remain in the fibers produced by these methods, which similar to wet spinning, necessitate the use of a coagulation bath to promote fiber formation and remove the residual ionic liquids. Ethanol baths have been used for this purpose.
Another approach to processing cellulose involves dissolving modified cellulose derivatives for electrospinning. A representative example is cellulose acetate, where solvent selection is critical. Ma et al. reported that cellulose acetate could be electrospun from a solution dissolved in a mixed solvent of acetone, DMF, and trifluoroethanol (TFE)22. Since DMF has a high boiling point, the addition of TFE and acetone increased volatility, improving spinnability and fiber formation stability. Cheng et al. used a mixed solvent of acetone and dimethylacetamide for electrospinning under high humidity, forming a three-dimensional nanofiber stack23. This study also demonstrated that solvent volatility affects fiber morphology.
Zhang et al. proposed a method to oxidize cellulose using periodate, making it water-soluble and allowing electrospinning with only water as the solvent, thereby avoiding harmful organic solvents. After fiber formation, chemical cross-linking was performed to obtain water-insoluble fibers24.
Although cellulose itself is poorly soluble in most solvents, the use of cellulose nanofiber (CNF) suspensions has been explored as an alternative approach. CNF is derived from the fibrillation of wood or pulp and is a renewable biomass material with excellent properties, such as high strength and low weight. CNF is particularly known for its high tensile toughness, and when aligned and incorporated into composites, it is expected to produce materials stronger than conventional ones.
While CNF disperses well in water, electrospinning CNF suspensions presents significant challenges. At low concentrations, the viscosity is too low to form the Taylor cone at the nozzle tip, which is essential for electrospinning. Conversely, at high concentrations, the suspension becomes gel-like and cannot be ejected from the nozzle. To address this, water-soluble polymers have been added as support polymers to facilitate electrospinning.
Enayati and colleagues produced fibers by adding CNF to PVA and electrospinning the mixture25. In this case, CNF acted as a filler to reinforce the PVA fibers. The finding that CNF could be electrospun in its suspension state, even without being dissolved, was particularly intriguing. However, the resulting fibers did not meet expectations in terms of elastic modulus or elongation at break. It has been suggested that hydrogen bonding between cellulose and PVA may have disrupted the crystalline structure of cellulose, leading to these suboptimal results.
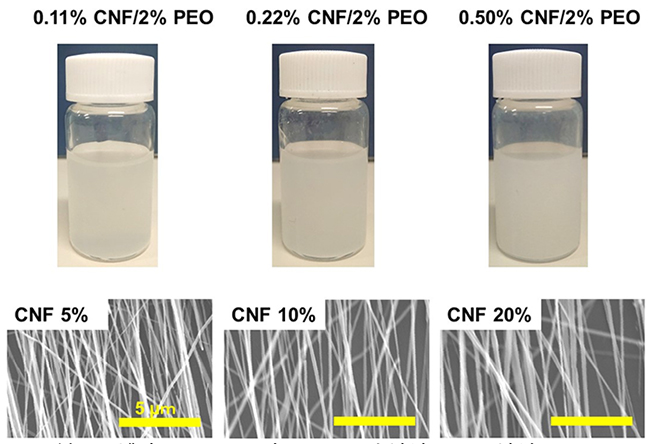
We successfully produced CNF-extended and bundled nanofibers by electrospinning CNF dispersions mixed with PEO26 (Figure 5). This approach resulted in fibers with high mechanical strength.
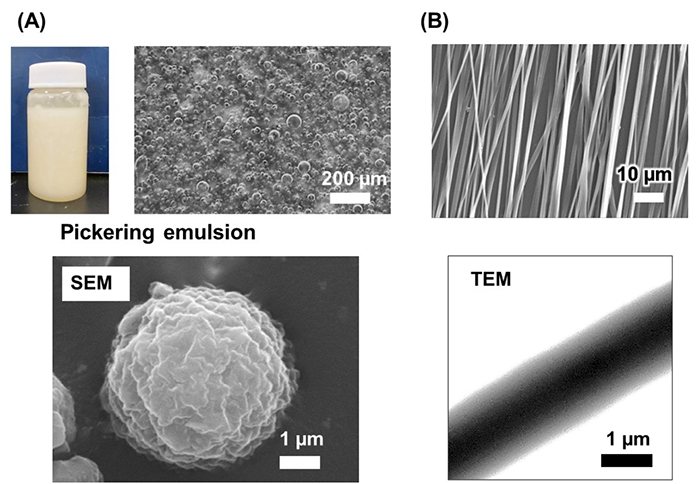
While CNF disperses well in aqueous suspensions, dispersing it in hydrophobic solvents or polymers is difficult. As a result, incorporating CNF into hydrophobic polymers has proven challenging. We focused on Pickering emulsions, which are stabilized by solid particles27. By dissolving the hydrophobic, biodegradable polymer poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) in chloroform and mixing it with a CNF water suspension, we successfully created a Pickering emulsion stabilized by CNF. Through direct electrospinning of this emulsion, we produced fibers in which CNF was extended and bundled within the PHBH fiber matrix (Figure 6).
Dextran is a water-soluble polysaccharide composed of glucose units primarily linked by α-1,6 bonds, with branching points. It is widely used in pharmaceutical practice as a plasma substitute, and in the food and cosmetics industries as a thickening and stabilizing agent. Jiang et al. produced dextran membranes via electrospinning and evaluated their properties28. The characteristics of dextran can be tuned by selecting appropriate solvents and additives. For example, when water is used as a solvent, proteins such as lysozyme can be directly incorporated, whereas DMSO/DMF solutions enable the production of composite membranes with poly (lactic-co-glycolic acid). Additionally, methacrylated dextran can undergo photopolymerization to create durable membranes, which are expected to have applications in drug delivery and tissue engineering.
Borteh and colleagues fabricated electrospun scaffolds using acetalated dextran (Ac-DEX) and evaluated their ability to provide sustained release of therapeutic agents29. Ac-DEX is a modified for of dextran in which the hydroxyl groups of the glucose backbone are replaced by acetal groups. The hydrolysis rate of Ac-DEX can be adjusted by controlling the ratio of cyclic to acyclic acetals. Acyclic acetals decompose rapidly, while cyclic acetals decompose more slowly, allowing for controlled scaffold degradation. They incorporated the immunomodulatory drug resiquimod into the scaffold and demonstrated that the drug release rate depended on the scaffold's degradation speed. Drug release was rapid from faster-degrading scaffolds, while more gradual from slower-degrading scaffolds.
Dextrin is a polysaccharide derived from the hydrolysis of starch, primarily composed of glucose units linked by α-1,4 bonds with branching through α-1,6 bonds. When glucose units link in a cyclic structure, they are known as cyclodextrins, which are widely used in the food and pharmaceutical industries. Celebioglu et al. reported the direct production of nanofibers from low-molecular-weight sugars using electrospinning with hydroxypropyl-β-cyclodextrin (HPβCD)30. HPβCD exhibits high viscosity due to self-aggregation, allowing for stable nanofiber formation without the need for additional polymers. Furthermore, the addition of urea suppressed self-aggregation, resulting in a reduction in fiber diameter and the formation of bead-like structures. Bead-free nanofibers were also obtained using the inclusion complex of HPβCD with the antimicrobial agent triclosan (HPβCD/triclosan-IC), and the retention of the inclusion complex was confirmed by various analytical methods.
This article has reviewed the use of polysaccharides and their derivatives in electrospinning. Electrospun polysaccharide nanofibers are expected to find applications across a wide range of fields, including wound healing, tissue engineering, and drug delivery, particularly as anisotropic hydrogel fibers. Future advancements in the functionalization of these materials and the development of novel composites are anticipated, leading to more sophisticated applications. Additionally, due to safety concerns, improvements in the solvents and cross-linking methods continue to be highly sought after. Three-dimensional scaffold materials based on these technologies are expected to make significant contributions in areas such as artificial organs, tissue models for drug evaluation, regenerative medicine, and drug discovery.
The work presented here includes the result of collaboration works with Professor Shin-ichiro Suye (University of Fukui, Japan), many other professors, and my students, to whom I express my sincere gratitude.