
Tsutomu Suzuki
Professor, Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo
Also holds positions in the Department of Bioengineering and the Institute for Quantitative Biosciences, The University of Tokyo
He completed Ph.D. program in School of Life Science and Technology, Tokyo Institute of Technology in 1996, worked at the Yokohama Research Laboratory of Mitsubishi Chemical Corporation, and was appointed assistant professor at the University of Tokyo in 2004, and was promoted to professor in 2008. From 2020, he has been appointed as Research Director of the JST-ERATO SUZUKI RNA Modification Project. His research motto is to uncover the molecular aspects of biological phenomena. His daily routine is to swim a mile in the pool on weekdays.
Transfer RNA (tRNA), an adaptor molecule in protein synthesis, contains various types of chemical modifications that play crucial roles in its function. Queuosine (Q), characterized by a 7-deazaguanosine bearing a bulky side chain with a cyclopentene group, is a tRNA modification widely present in both bacteria and eukaryotes. In the tRNAs of humans and vertebrates, Q can be further galactosylated to form galactosyl-queuosine (galQ), or mannosylated to form mannosyl-queuosine (manQ). The function of these glycosylated Q modifications had remained unclear for nearly half a century since their discovery. We recently identified two glycosyltransferases responsible for the formation of galQ and manQ, and have elucidated the biosynthesis, functions in protein synthesis, and physiological significance of these glycosylated Q tRNA modifications.
RNA molecules undergo various types of chemical modifications post-transcriptionally, maturing and acquiring diverse cellular functions. While DNA, another nucleic acid, has been found to have only a few species of modified bases, approximately 150 types of RNA modifications have been identified across all domains of life to date. These modifications exhibit a wide range of chemical variations, including methylation, acetylation, phosphorylation, hydroxylation, dehydration cyclization, sulfurization, selenation, reduction, isomerization, and the addition of amino acids or sugars to the bases or riboses1. It is believed that these diverse RNA modifications were acquired during evolution to enable RNA to fulfill new functions. In recent years, several groups, including ours, have reported novel RNA modifications, and the chemical space of RNA modifications continues to expand2-4. Moreover, it is known that deficiencies in RNA modifications cause human diseases, proposing a new concept of human disease caused by aberrant RNA modification, termed as ‘RNA modopathy’4-7. The field of epitranscriptomics is rapidly gaining global attention as a promising target for drug discovery and therapy, with a growing number of specialized research initiatives and biotech startups emerging in this area, attracting considerable interest from the industry.
tRNA serves as an adaptor molecule in protein synthesis, converting codons on mRNA into corresponding amino acids. tRNA is the most extensively modified RNA molecule, with approximately 80% of known RNA modifications identified in tRNAs2. In particular, a wide variety of modifications are found at the first nucleotide (position 34) of the anticodon (Figure 1A), enabling tRNA to accurately and efficiently decipher codons on mRNA at the A-site of the ribosome during translation. Queuosine (Q) is a unique tRNA modification characterized by a 7-deazapurine core structure with a cyclopentene ring, found in a broad range of organisms from bacteria to humans (Figure 1B). Curiously, bacteria are able to synthesize the base of Q, called queuine (q), whereas humans and other vertebrates cannot synthesize q de novo. Instead, they acquire q as a dietary nutrient and/or from gut microbiota, and use it for tRNA Q modification8 (Figure 1B). In humans, the QTRT1 and QTRT2 heterodimer complex exhibits transglycosylase activity, and replaces the guanine base of the first letter of the tRNA anticodon with q, thus forming Q on the tRNA. Four types of cytoplasmic tRNAs for histidine (His), asparagine (Asn), tyrosine (Tyr), and aspartic acid (Asp) carry the Q modification in humans and vertebrates. Of these, Q is further galactosylated to form galactosyl-queuosine (galQ) in tRNATyr, while Q is mannosylated to form mannosyl-queuosine (manQ) in tRNAAsp (Figure 1B). Since their discovery in 1976 by Dr. Susumu Nishimura and his colleagues9, galQ and manQ have been intriguing research subjects as rare glycosylated tRNA modifications. However, their biosynthesis and functions remained elusive for nearly half a century.
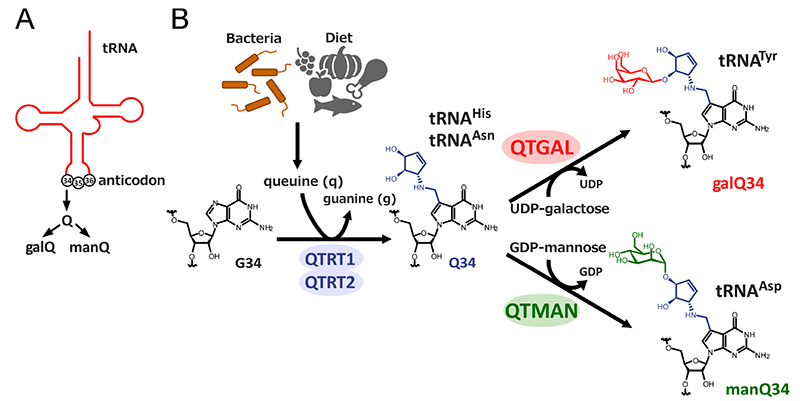
Glycosyltransferases are a large group of enzymes that transfer a sugar moiety from an activated sugar donor onto various acceptors, including proteins and lipids. Approximately 230 glycosyltransferase genes are encoded in the human genome10,11. Our research group initially attempted to identify the enzymes responsible for galQ and manQ modifications by using comparative genomics to search for glycosyltransferases with unknown function, but these efforts were unfruitful. Fortunately, we succeeded in detecting enzymatic activities from cell lysates to reconstitute galQ on tRNA using UDP-galactose, and manQ on tRNA using GDP-mannose. Using those enzymatic activities as indicators, we biochemically concentrated the glycosyltransferases for galQ and manQ. By fractionating rat liver lysate using ammonium sulfate precipitation and multiple column chromatographies, we successfully identified the enzyme B3GNTL1, which transfers galactose to the Q-modified tRNATyr. We named this enzyme queuosine tRNA-galactosyltransferase, QTGAL (Figure 1B)12. Additionally, we fractionated pig liver lysate and identified the enzyme GTDC1, which transfers mannose to the Q-modified tRNAAsp, and named it queuosine tRNA-mannosyltransferase, QTMAN (Figure 1B)12.
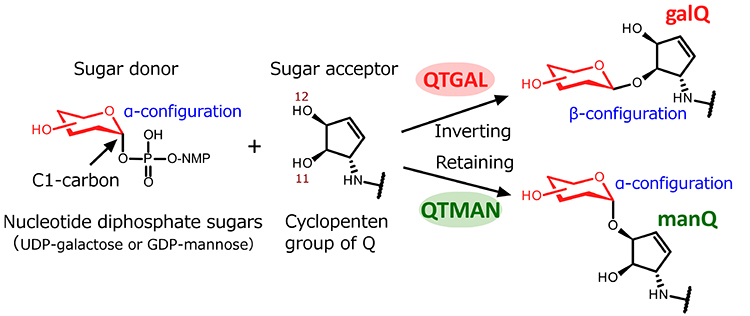
QTGAL and QTMAN synthesize galQ and manQ, respectively, using UDP-galactose and GDP-mannose as sugar donors. Sugar transfer reactions are classified by two types in terms of inversion or retention of the stereochemistry at the C1 anomeric carbon of the sugar donors, converting it from an α to a β configuration or retaining the α configuration (Figure 2). QTGAL belongs to the GT2 family bearing a GT-A fold, and catalyzes an SN2-type nucleophilic substitution at the hydroxyl group of the C11 carbon of the cyclopentene group of Q. This reaction inverts the stereochemistry of the C1 carbon of UDP-galactose from α to β configuration, resulting in the formation of galQ (Figure 2). On the other hand, QTMAN belongs to the GT4 family characterized by a GT-B fold, and catalyzes a retention-type sugar transfer reaction to form manQ. This reaction retains the α configuration of the C1 carbon of GDP-mannose to transfer mannose to the hydroxyl group of the C12 carbon of the cyclopentene group of Q (Figure 2).
Kinetic analyses revealed that the Michaelis-Menten constant (Km) of QTGAL for UDP-galactose was 14 μM, while the Km value of QTMAN for GDP-mannose is relatively high at 69 μM. The intracellular concentrations of UDP-galactose and GDP-mannose are estimated to be 7-65 μM 11,13-16 and 0.9-11 μM13, respectively. These Km values are in a similar range of the intracellular concentrations of the sugar donors, suggesting that Q-glycosylation might be regulated by the intracellular concentrations of UDP-galactose and GDP-mannose.
We constructed human knockout cells lacking QTGAL or QTMAN gene (QTGAL KO or QTMAN KO), and confirmed the absence of galQ and manQ in the tRNAs isolated from each of these KO cells. Collaborating with Iwasaki lab. at RIKEN, we conducted ribosome profiling experiments, and observed the increased translation speed of the UAC codon read by tRNATyr in QTGAL KO. Similarly, we found that the upregulation of both GAC and GAU decoding by tRNAAsp in QTMAN KO cells. Since Q modification in tRNAs generally accelerates the translation speed of their cognate codons, these findings suggest that the Q-glycosylations are negative regulators to modulate decoding speed for optimal translation. In general, tRNA modifications typically contribute to efficient translation. These findings are the first instance of tRNA modifications which slow down translation speed.
We examined the effect of galQ on stop codon readthrough, because tRNATyr is a kind of natural suppressor for stop codons. Using a dual luciferase reporter to measure stop codon readthrough, we observed that galactosylation of galQ prevents the readthrough of UAG and UAA codons. This is an important function of galQ in protein synthesis.
tRNA modifications generally contribute to optimal translation, thereby maintaining proteostasis. The absence of tRNA modifications is known to cause misfolding of nascent proteins17. We used an aggregation-prone reporter, and clearly observed protein aggregation in a series of KO strains. So, protein folding is impaired by loss of Q-glycosylations. In other words, Q-glycosylations have function to maintain proteostasis.
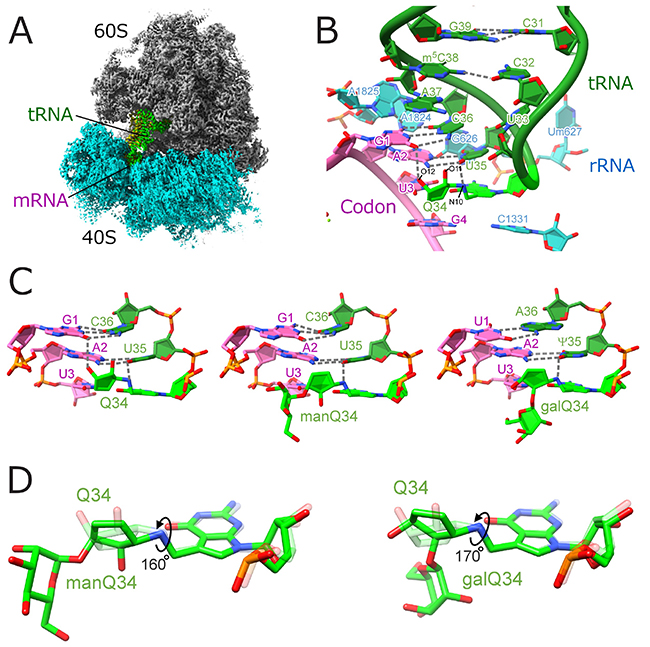
To visualize structural details of codon–anticodon interactions mediated by Q and glycosylated-Q, we performed cryo-EM single-particle analysis of human 80S ribosome complexed with tRNAs and mRNA, with the support of the Shirouzu lab. at RIKEN. We obtained high resolution structures of ribosome complexes with different tRNAs and codons (Figure 3AB). The resolution was good enough to model the tRNA modifications. The cyclopentene group of the Q modification binds to the major groove of the codon-anticodon duplex via hydrogen bond network, functioning as a major groove binder and thereby enhancing decoding efficiency (Figure 3C). This effect was particularly prominent in U-ending codons, UAU and GAU. On the other hand, we found that Q-glycosylations attenuate this effect by rotating the cyclopentene ring (Figure 3D), revealing the molecular basis of translational regulation by glycosylated Q modifications.
To explore the physiological function of Q-glycosylation, we collaborated with Mishima lab. in Kyoto Sangyo University, and constructed genetic mutants of zebrafish for qtgal, and qtman. All mutants are viable and exhibit normal embryonic development, but show a slower postnatal growth with shorter body length compared to the wild-type zebrafish. Furthermore, in the qtgal KO line, we observed a modest increase of eIF2α phosphorylation, suggesting that impaired translation rate by loss of Q-glycosylation leads to protein misfolding, triggering the integrated stress response. These findings indicate that glycosylated Q modifications of tRNAs are essential for the healthy growth of zebrafish.
Human disease relevance of Q modification has been reported18. It has been observed that Q is reduced in malignant tumors and cancer cells19-21. Curiously, hypomodification of Q strongly correlates with the stages of various cancers22. Moreover, the deficiency of QTRT1 inhibits the cancer cells proliferation23. According to the TCGA database24, QTRT1, QTRT2, QTMAN, and QTGAL are highly expressed in many tumors. High expression of QTRT1 is associated with poor prognosis in renal cancer, while it shows the opposite trend in cervical cancer. QTRT2 expression correlates with worse prognosis in brain tumors. High expression of QTGAL is linked to poor prognosis in renal cancer. QTMAN shows a complex pattern. Low expression of QTMAN is associated with poor prognosis of brain tumors, whereas its high expression correlates with poor prognosis in liver cancer. Beyond cancer, dysfunction of GTDC1 (QTMAN) has been suggested to cause neurodevelopmental disorders by leading to excessive activation of NMDA receptors through glycine metabolism abnormalities25. Additionally, the loss of GTDC1 (QTMAN) in neural progenitor cells (NPCs) and neurons showed phenocopy with iPS cells derived from patients with neurodegenerative diseases26.
In this study, we identified two tRNA glycosyltransferases both of which are members of the glycosyltransferase superfamily, leaving many unanswered questions about how these enzymes, which typically glycosylate proteins and lipids, evolved to specifically recognize tRNA molecules. We are currently studying physiological roles of Q-glycosylation using Qtgal and Qtman knockout mice. In the future, we aim to elucidate the physiological impact of Q-glycosylation in mammals and its association with human diseases. Recently, glycoRNAs, sugar chain modifications of RNA molecules, have been discovered27, expanding a research field of RNA and glycosylation. It will be exciting to see what discoveries lie ahead as the worlds of glycosylation (the glycome) and RNA modification (epitranscriptomics) converge, and we will continue to closely follow the development of this emerging field.
This research began with the identification of QTGAL and QTMAN by Xuewei Zhao and Ding Ma, who were our former graduate students. The structural analyses of tRNAs with glycosylated Q modification using cryo-EM was conducted by Kensuke Ishiguro, a project assistant professor of JST-ERATO project. I would like to take this opportunity to express my deep gratitude for their invaluable contributions. Finally, I would also like to extend my sincere thanks to Takeo Suzuki (currently a professor at the University of the Ryukyus, School of Medicine), who has long been a key member of our team and greatly contributed to this project.