
Kazuhiro Fukada
Professor, Faculty of Agriculture, Kagawa University
Kazuhiro Fukada graduated from Department of Chemistry, School of Science, Nagoya University in 1983, and completed the doctoral course of the Graduate School of Science, Nagoya University in 1989 (Doctor of Science). He became an assistant professor at Department of Chemistry, Faculty of Science, Tokyo Metropolitan University in 1989, an associate professor at the Faculty of Agriculture, Kagawa University in 2001, and a professor there in 2005.
His specialties are colloid science and biophysical chemistry. He has studied the physicochemical properties of biological substances (e.g., lipids) and surfactants. After his appointment at Kagawa University, he participated in research on rare sugars to investigate their molecular structure and thermodynamic properties, and has been involved in this field for more than 20 years. He is interested in how the laws of physical chemistry can explain the complex phenomena that occur in our daily life.

Ken Izumori
Research Advisor, International Institute of Rare Sugar Research and Education, Kagawa University; Professor Emeritus, Kagawa University
Background: Ken Izumori graduated from Department of Agricultural Chemistry, Faculty of Agriculture, Kagawa University in 1965, and became assistant professor at the Faculty of Agriculture, Kagawa University in 1968. After serving as an associate professor and professor there, he became a professor at the Rare Sugar Research Center, Kagawa University in 2003. In 2008, he became visiting professor at Kagawa University, as well as professor emeritus at Kagawa University and specially appointed professor at the Faculty of Agriculture, Kagawa University. Since 2016, he has served as research advisor at the International Institute of Rare Sugar Research and Education, Kagawa University.
His specialty is microbial utilization. He says he has realized the importance of collaboration with researchers in different fields.
The previous article of this series described what rare sugars are (the definition of rare sugars and their roles in nature) and the history of rare sugars (a scenario of rare sugar formation on the Earth and the history of rare sugar research). Rare sugars are defined as monosaccharides and their derivatives rarely found in nature. Commonly known monosaccharides range from trioses (with three carbon atoms) to heptoses (seven carbon atoms). This article series focuses mainly on monosaccharides with six carbon atoms, namely hexoses. Many rare sugars with interesting bioactivity have six carbon atoms. The reason for this is difficult to explain, but it may be related to the fact that organisms mainly metabolize D-glucose and other hexoses.
In this second article of the series, we describe the development history of the strategy map for the systematic production of all hexoses (Izumoring), the significance of the production strategy map, problems that have become apparent in Izumoring-based rare sugar production, and a new strategy map for rare sugar production (Izumoring Ⅱ) developed to solve these problems. We also introduce a simplified version of the Fischer projection formula for monosaccharides (Izumofleet formula), which is used in the new strategy map for rare sugar production.
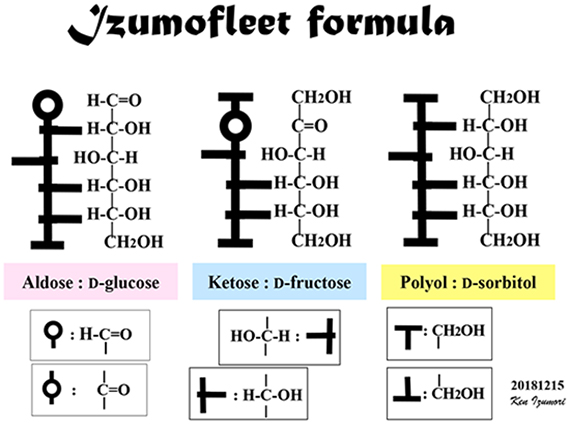
First, we show the variety of hexoses and polyols (hexitols); the latter are produced by the reduction of hexoses. To assist in understanding, the following figures of this article show the structures of these molecules in Fischer projection, with red, dark blue, and yellow circles, representing aldoses, ketoses, and polyols, respectively (Fig. 1). Here we show the Fischer projections of an aldose D-glucose, ketose D-fructose, and polyol D-sorbitol (= D-glucitol); the asymmetric carbons in each molecule are marked with asterisks (*).
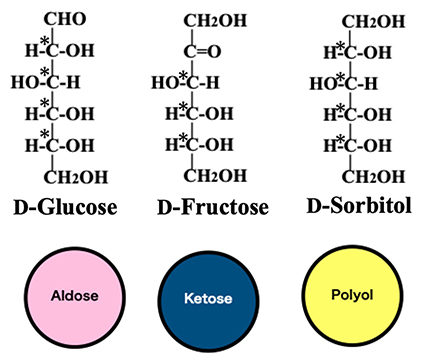
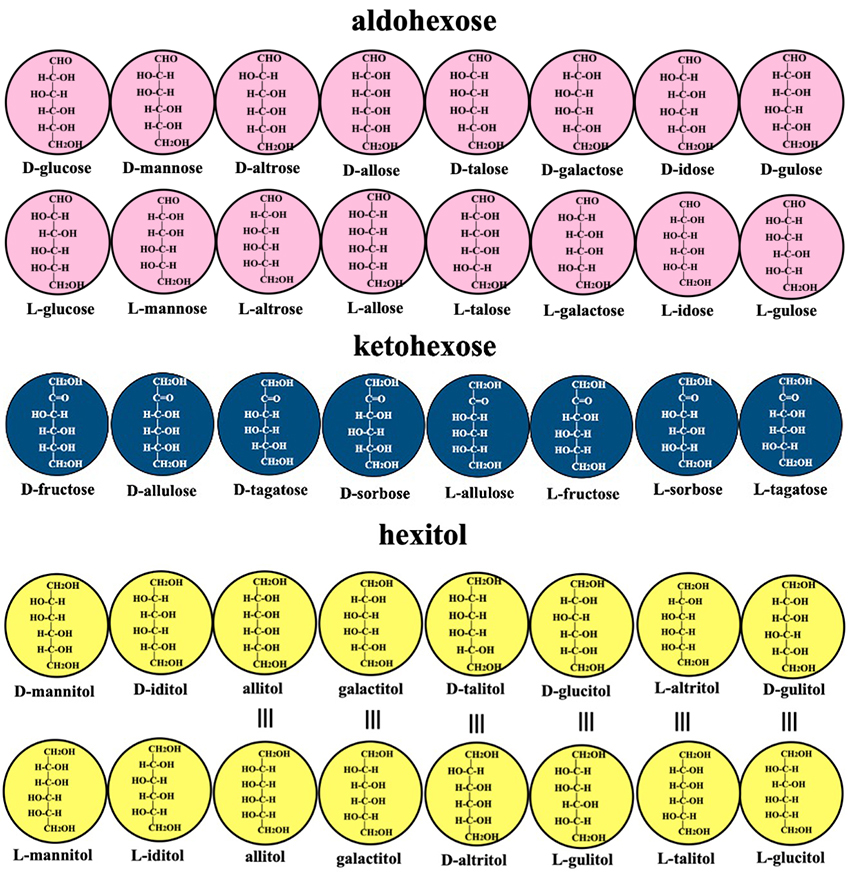
As can be seen in Fig. 1, aldohexoses have four asymmetric carbons and therefore exist in 16 (= 24) different isomeric forms. Ketohexoses have three asymmetric carbons and therefore form 8 (= 23) different molecules (see Fig. 2). Since hexitols have four asymmetric carbons, it would seem that there are 16 (= 24) forms, but in fact only 10 (= 16–6) exist, because six pairs of isoforms are identical substances, including the meso compounds allitol and galactitol, or D-glucitol and L-gulitol (see the pairs of yellow circles in the upper and lower rows with ≡ between them in Fig. 2). Totally, there are 34 different molecules; all except D-glucose, D-mannose, D-galactose, and D-fructose are classified as rare sugars; that is, most hexoses and hexitols are rare sugars.
Next, we introduce our basic approach to creating the strategy map for the comprehensive production of all these hexoses and hexitols, and the path we have taken to complete the map.
The basic approach to establishing the comprehensive production method for rare sugars (hexoses and hexitols) is as follows: D-glucose is chosen as the starting material because of its availability in large amount at low cost; D-glucose is sequentially converted to other hexoses by enzymatic reactions; finally, the relationships between hexoses are defined based on the enzymatic reactions. We considered that summarizing these reaction pathways would provide a strategic map for the systematic production of rare sugars. The important point here is what kinds of specific conversion reactions lead to a variety of hexoses. We describe below the enzymatic reactions used in the production of rare sugars.
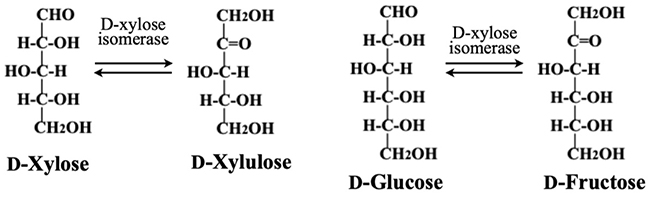
First, we would like to explain the process of manufacturing isomerized sugar, which is mass-produced as a sweetener. D-glucose can be produced at low cost by hydrolyzing starch. However, as the relative sweetness of D-glucose compared to sucrose is as low as 0.6-0.7, D-glucose alone has limited value as a sweetener. On the other hand, D-fructose, which is produced by isomerization of D-glucose, has a relative sweetness approximately 1.2-1.5 times higher than that of sucrose. Therefore, isomerized sugar, which is a 1:1 mixture of D-glucose and D-fructose, is mass-produced from D-glucose by isomerization as a less expensive sweetener than sucrose. In vivo, however, free D-glucose is not metabolized directly but is first phosphorylated and then isomerized to D-fructose. In other words, no enzyme in vivo isomerizes free D-glucose. The enzyme used in the production of isomerized sugar is microorganism-derived D-xylose isomerase. Although its native substrate is D-xylose, this enzyme also acts on free D-glucose because of the structural similarity between D-xylose and D-glucose; this enzymatic property is used to produce isomerized sugar (Fig. 3).
Such an isomerization reaction is also possible between the two hexoses D-galactose and D-tagatose. The metabolism of D-galactose in vivo is mainly via UDP-D-galactose, and living animals, including humans, do not have any enzymes that isomerize free D-galactose to D-tagatose. However, L-arabinose isomerase produced by microorganisms acts on free D-galactose and converts it to D-tagatose.
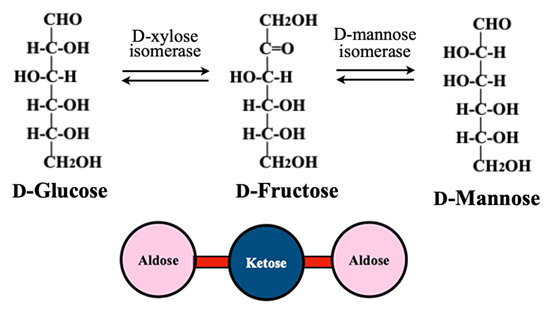
Isomerization between an aldohexose and a ketohexose is an equilibrium reaction, and a ketohexose can be converted to two aldohexoses by selecting an appropriate isomerase. For example, D-fructose is converted to D-glucose by D-xylose isomerase, or isomerized to D-mannose by D-mannose isomerase (Fig. 4). Similarly, by using isomerases, each of the eight ketoses can be converted to two aldoses; as a result, eight different blocks of relationships can be made.
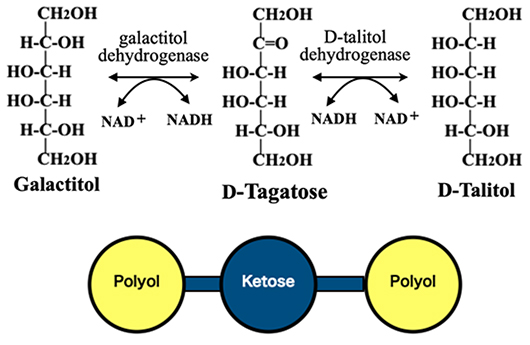
As mentioned in the previous article of this series, Bertrand demonstrated that D-sorbitol (= D-glucitol) is converted to L-sorbose by the action of acetic acid bacteria. This is a D-sorbitol dehydrogenase (acceptor)-catalyzed oxidation reaction that converts the polyol to the ketohexose. We also mentioned that, by mimicking this reaction, a rare sugar, D-tagatose, was produced from galactitol. The reaction catalyzed by dehydrogenase is reversible, as is the isomerization reaction catalyzed by isomerase, and the reduction of ketose to polyol is also possible. In addition, two different polyols can be produced from a single ketohexose by selecting an appropriate dehydrogenase. An example of such a reaction is shown in Fig. 5: the reaction that produces galactitol and D-talitol from D-tagatose. In this way, all eight ketoses are related to two polyols.
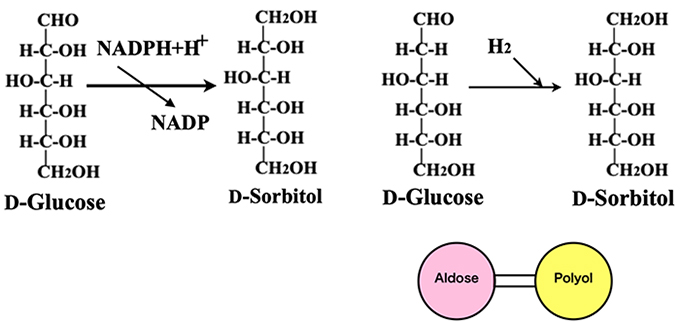
Aldose reductase is known to catalyze the reduction of aldehyde groups of aldohexoses to produce polyols. Figure 6 shows the reduction of D-glucose to D-sorbitol (= D-glucitol). This enzymatic reaction requires the coenzyme NADP and is not suitable for factory-scale production. To reduce large amounts of D-glucose, Raney nickel, instead of the enzyme, is used the catalyst of hydrogenation with hydrogen gas (Fig. 6, right). In this way, we can relate aldoses and polyols. This reaction can reduce the substrate D-glucose to the polyol with a 100% yield and also can be used for the reduction of ketoses as well as aldoses.
Most of the reactions described above are already known enzymatic reactions with monosaccharides. We have also stated that all ketoses are related to two aldoses and two polyols by enzymatic reactions with native substrates and structurally similar hexoses, and in addition, that aldoses can be converted to polyols by reduction. Is it then possible to relate all 34 hexoses and hexitols by these reactions? Unfortunately, this is not possible at this point. Something is still missing.
The basic goal of the rare sugar production strategy, “defining the relationships between the hexoses based on enzymatic reactions,” cannot be achieved by the enzymatic reactions introduced so far. This situation was changed by the discovery of the enzyme D-tagatose 3-epimerase. This enzyme epimerizes the 3-position of D-tagatose and converts it to the corresponding epimer (Fig. 7). Moreover, D-tagatose 3-epimerase was found to be active against all eight ketoses. The emergence of this new enzyme opened up the possibility of establishing relationships between hexoses based on enzymatic reactions.
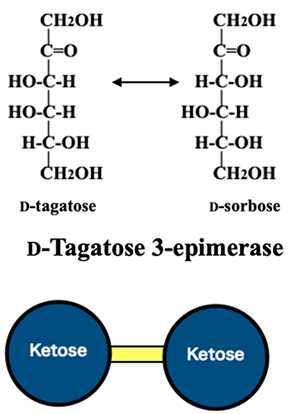
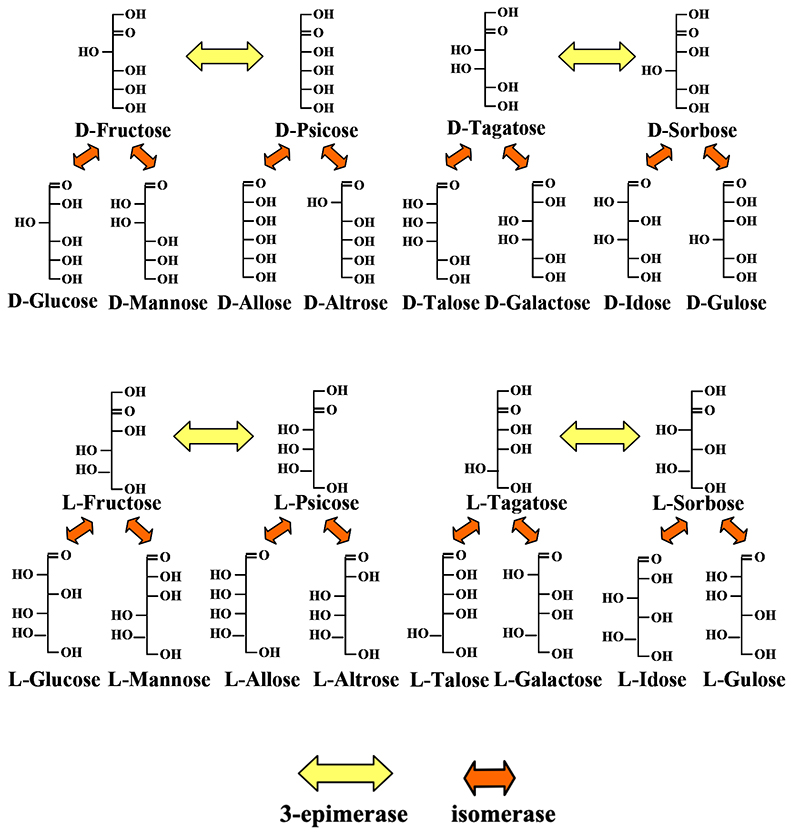
Fig. 8 lists the isomerase- and epimerase-mediated conversions between all the D- and L-form aldohexoses and ketohexoses. These reactions could be grouped into four blocks with two ketoses and four aldoses as a unit, but we could not relate the blocks to each other. We needed an idea to fill this gap.
The number of hexoses and hexitols is only 34 in total. Having recalled the structures of hexoses and hexitols, enzymatic reactions were considered, starting from D-fructose, in the way of a professional shogi (Japanese chess) player playing a game without a board. Then, a circular reaction pathway that returns to the original D-fructose came to mind, and the idea was immediately put on paper; the schematic drawing at that time was a ring consisting of eight ketoses relate ed via polyols (Fig. 9). It is important here to note that four identical polyols are produced by the reduction of different ketoses, as shown in Fig. 2 (L-gulitol and D-glucitol, D-altritol and D-talitol, D-gulitol and L-glucitol, L-altritol and L-talitol).
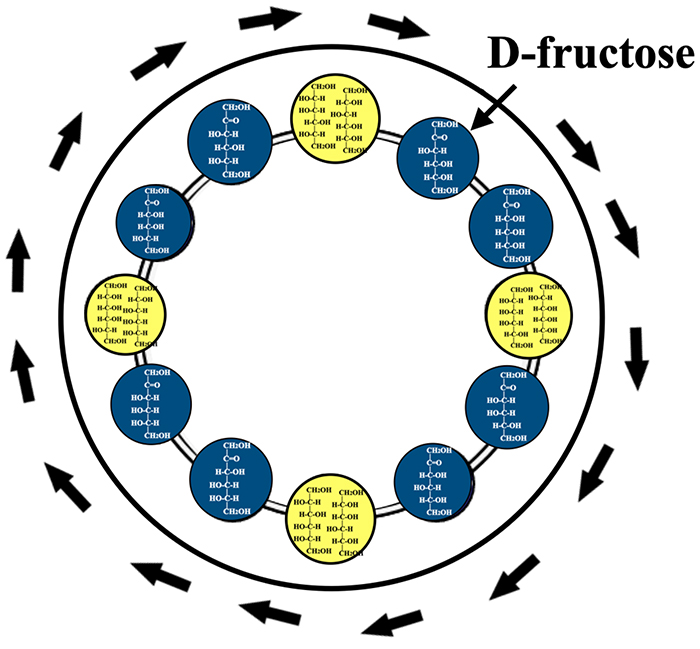
As depicted in Fig. 9, epimerase-mediated conversions between ketoses and dehydrogenase-mediated redox reactions relate ketoses and polyols sequentially to make a circular pathway starting from and ending in D-fructose1. When we hear the word hexose, we first think of D-glucose or D-fructose. Moreover, since we initially assumed that D-glucose was the starting material, we focused on the conversion between aldoses and ketoses and did not consider the conversion pathway between ketoses and polyols. As all the eight ketoses are included in Fig. 9, it seems possible, based on this ring, to create a pathway in which all hexoses are related together.
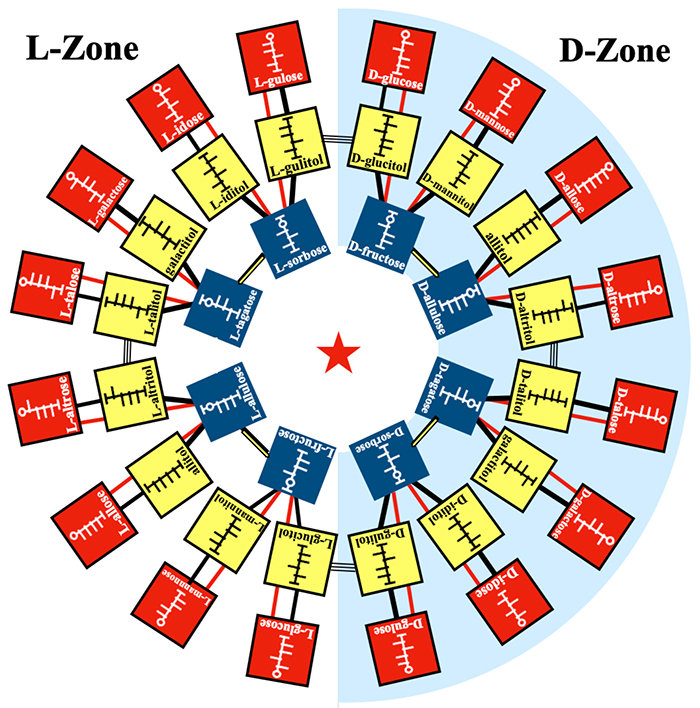
Based on the ring in Fig. 9, which consist of ketose ⇄ polyol ⇄ ketose ⇄ ketose ⇄ …, we considered the interconversions with other aldoses and polyols and the arrangement of these sugars; aldoses were placed outside the ring, whereas polyols inside. By this arrangement, we established the relationship of all hexoses and hexitols based on enzymatic reactions. Fig. 10 shows the complete map2,3.
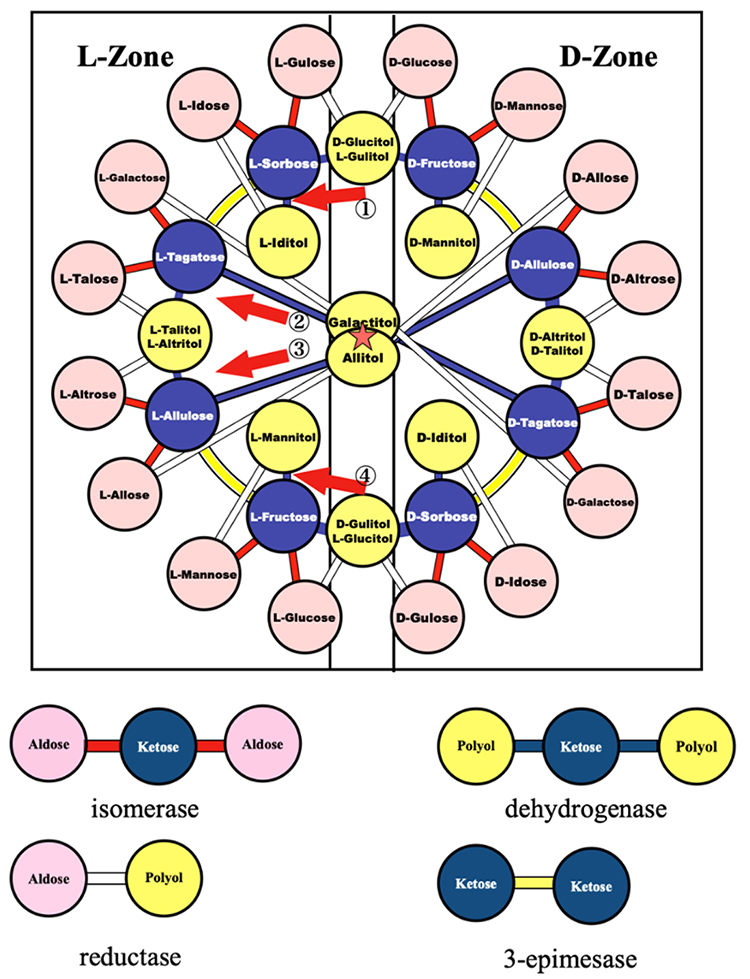
When we first saw this map, we found it to be “beautiful” (though perception may not be scientific). We summarize the features of this map below.
The completed strategy map was named the Izumoring by a student in our laboratory. We kept this name in the submitted paper, expecting that the reviewers would ask us to change it to a more appropriate name. We thought a term that evokes an individual's name (Izumori) must be considered inappropriate. However, the manuscript was accepted without any comment on the term Izumoring, although there were some requests for revising the English text1. Subsequently, when asked about the origin of the word Izumoring abroad, we explain that “In Japan, there is a place called Izumo where all the gods in the country gather once a year. The term Izumoring is a combination of the words ‘Izumo’ and ‘ring’.”
The Izumoring in Fig. 10 shows a ring-shaped map, in which all the hexoses with six carbon atoms are interrelated each other based on enzymatic reactions. Similar rings can also be drawn for tetroses and pentoses and provide blueprints for the production of rare sugars with four and five carbons, respectively2.
We should definitely mention that in the process of completing the Izumoring, we were very lucky. That is, when considering the structure of monosaccharides, we continued to use the Fischer projection formula. Few sugar researchers use Fischer projections to draw monosaccharides because the actual monosaccharide structure is not a straight chain (Fischer projection) but mostly cyclic, as represented by pyranose and furanose forms. The Fischer projection formula is used only in very limited cases, such as when comparing the stereo configuration of hydroxy groups within a monosaccharide. However, during the development of the Izumoring, we illustrated the structural transformation of monosaccharides using Fischer projection formulas, which are representations of, so to speak, “the silhouette of the sugar molecule.” If we had not represented monosaccharide structures as Fischer projections, we would not have been able to complete the Izumoring, because the cyclic structures of monosaccharides are too complex to imagine a ring-shaped reaction pathway.
X-ray crystallography of enzymes that promote isomerization or epimerization of monosaccharides has shown that monosaccharides in solution form cyclic structures, but binding to these enzymes opens the rings and they become chain-form reaction intermediates4,5. Considering this reaction mechanism, it was reasonable to use the Fischer projection formula to depict monosaccharide structures for the purpose of interrelating a series of structural transformations.
After the Izumoring was established, we found a very interesting paper on a microorganism that degrades the rare sugar L-glucitol. The microorganism had the following metabolic pathway: L-glucitol→D-sorbose→D-tagatose→D-galactose. This is definitely the pathway found in the Izumoring. Converting L-glucitol to D-galactose by this pathway and further metabolizing D-galactose via the De Ley-Doudoroff pathway is the survival strategy of this microorganism. The Izumoring was originally developed as a strategy to produce rare sugars, but it turned out that microorganisms in nature also use the Izumoring pathway6.
We previously investigated the degradability of rare sugars by the aeration method, assuming an accidental release of large amounts of rare sugars into the environment when they are produced on a large scale. Rare sugars were added to the water samples collected from the aeration tank at the sewage treatment facility in our university; the samples were aerated using an air pump, and the changes in the concentration of rare sugars were measured over time. As a result, the concentration of rare sugars decreased in all cases examined, albeit at a slow rate in some cases depending on aeration conditions. This finding suggests that rare sugars are degraded by microorganisms. In our experiments so far, all the examined rare sugars have been degraded. Although the details of the degradation pathway are not clear, the microbes in the aeration tank may also metabolize the rare sugars via the Izumoring pathway.
These facts make us think again about the still unknown metabolic pathways and the reason for the existence of rare sugars in nature.
The interconversions between monosaccharides shown in the Izumoring are equilibrium reactions in which the compositional formula (6H12O6) does not change even after the conversion, except for the redox reactions involving the polyols. Therefore, if the equilibrium constant (K) is determined by examining the sugar composition when the reaction reaches equilibrium, reaction Gibbs energy (ΔrG), a measure of the difference in thermodynamic stability between reactants and products, can be calculated from the following equation.
ΔrG=-RT ln K ⋯⋯⋯ (1)
In the equation, R is the gas constant and T is the absolute temperature. For example, in the reaction in which D-glucose (Glc) is used as a raw material and isomerized to D-fructose (Fru), the concentration ratio at equilibrium at 25°C is approximately 1 to 0.85 (i.e., K = 0.85). Based on this result, the reaction Gibbs energy for the isomerization of Glc to Fru can be calculated to be ~0.4 kJ mol-1, allowing quantitative evaluation of the difference in thermodynamic stability between the two sugars. By performing the same calculation sequentially for the interconversions between other monosaccharides in the Izumoring, thermodynamic stability can be compared among all the hexoses. Fig. 11 summarizes the equilibrium composition of the conversion reactions between hexoses determined to date, with Glc as the reference; the volume of each sphere represents its relative abundance at equilibrium. In this figure, the equilibrium compositions of the D- and L-forms are assumed to be the same in accord with the principle that “the thermodynamic properties of the enantiomers are the same,” and the D- and L-hexoses are placed in the same positions in the Izumoring. Note that in the Izumoring, allulose (Alu) and tagatose (Tag) can be interconverted by the redox reactions via allitol and tallitol, but the reaction pathway connecting the upper and lower blocks in Fig. 11 are not depicted because the equilibrium relationship for these reactions is unknown. However, since the ΔrG value between Gal (Sor) and Glc can be calculated from literature data7, and the equilibrium concentration of Gal (Sor) relative to Glc can also be calculated from the equation (1); therefore, the volume of each sphere reflects the relative abundance of the sugar it represents at equilibrium.
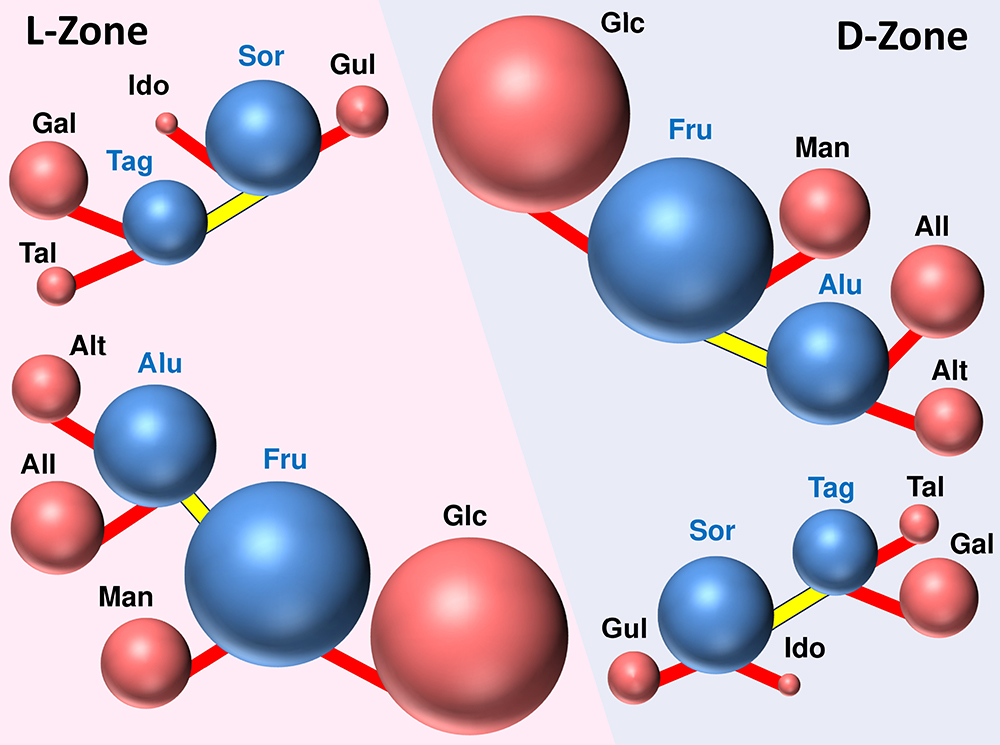
Fig. 11 reveals the following:
It is clear from these findings that the isomerase and epimerase reactions are very inefficient in producing various other hexoses from glucose, and the reactions yield only very small amounts of rare sugars. The reason for this is discussed below based on the molecular structure of hexoses in solution.
As mentioned above, hexose molecules do not exist in chain form as depicted by Fischer projections, but mostly in cyclic forms such as pyranose and furanose forms. Therefore, thermodynamic stability of hexoses must be discussed based on these molecular structures. Furthermore, it must be considered that the hexoses in solution are in a mixed state of totally five different tautomers coexisting in equilibrium; the five tautomers are the α- and β-anomers of pyranose and furanose forms, and a small amount of chain form.
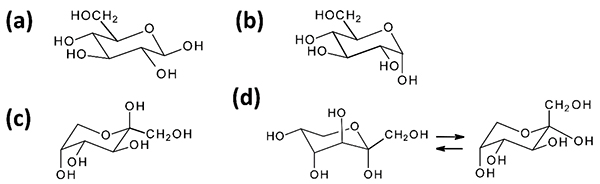
Fig. 12 shows the molecular structures of the α- and β-anomer of the pyranose forms of D-glucose and D-fructose. Of these, the β-anomeric form of D-glucose (β-D-glucopyranose) is considered a particularly stable structure because all four hydroxy groups are equatorial to the pyranose ring of the 4C1 conformation. In fact, 62% of the D-glucose in solution at 25°C is β-D-glucopyranose, and the remaining 38% is α-D-glucopyranose, which has an anomeric hydroxyl group in the axial position. On the other hand, the structure of β-anomeric D-fructose (β-D-fructopyranose) is the 1C4 pyranose ring that has a bulky CH2OH group in the equatorial position, with two axial and equatorial hydroxy groups each; this form of tautomer accounts for 64% of the abundance in solution. α-D-fructopyranose is unstable in structure and its abundance in solution is only 3%. This is because, when the pyranose ring is in the 4C1 conformation, the two hydroxyl groups are in the 1, 3-diaxial position, while in the 1C4 conformation, the CH2OH group is in the axial position. The furanose type, which is almost nonexistent for D-glucose, makes up 32%, and the chain type 1%.
Although fructose is a thermodynamically stable hexose along with glucose, it is not sufficient to only consider the pyranose ring when discussing the reason for its stability, because the structural stability of the furanose ring may also be involved. We thus summarized the equilibrium abundance ratios of tautomers in solution for all hexoses8,9,10 and examined their relationship to the thermodynamic stability (Fig. 13).
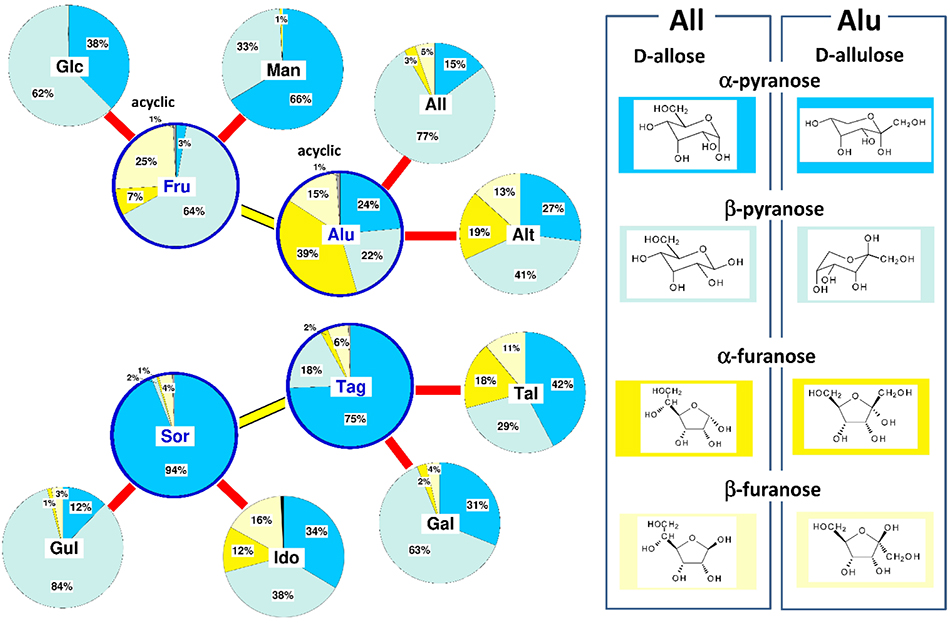
As has been shown in Fig. 12 ketohexoses other than fructose are also relatively stable; however, their tautomeric composition ratios are quite different. Allulose (Alu) is abundant in the furanose form, with α- and β-anomers together accounting for more than 50%. On the other hand, in tagatose (Tag) and sorbose (Sor), the α- and β-furanose forms together account for only less than 10%, and α-pyranose makes up the majority. This is because the 4C1 form of the pyranose ring in Tag and Sor is not structurally destabilized by the 1, 3-diaxial conformation of the hydroxy groups, making α-pyranose markedly more stable than other tautomers. Of the aldoses, mannose (Man), allose (All), and galactose (Gal) are moderately and similarly stable, with the furanose form accounting for less than 10% and the pyranose form predominating in all of these sugars. In the less stable aldoses (Alt, Tal, Gul, and Ido), more hydroxy groups are in the axial position and the pyranose ring becomes unstable; the furanose forms of all except gulose (Gul) accounts for up to 30% of the total.
Overall, the thermodynamic stability of hexoses seems to be determined by their stability in the pyranose ring form. As the number of axial hydroxy groups increases and the pyranose ring becomes unstable, the abundance of furanose tends to be higher. However, there are exceptions to this tendency. In particular, allulose (Alu), despite its relatively high stability, has a high abundance of furanose and differs markedly from other hexoses. This difference may affect the physicochemical properties and functionality of Alu.
By using the equilibrium constants for the conversion reactions between hexoses shown in the Izumoring, we were able to determine the thermodynamic stability of all hexoses, including rare sugars. The Izumoring was originally created as a strategy map for rare sugar production, but it has also been used to understand the basic properties of rare sugars and to clarify issues that need to be resolved in the production strategy.
By defining relationships between hexoses in term of enzymatic reactions, we were able to develop our strategy for the systematic production of rare sugars, but at the same time, a weakness of this methodology became apparent. That is, hexoses with low thermodynamic stability are difficult to produce because they can only be obtained in low yields in equilibrium reactions such as isomerization and epimerization. This issue is particularly problematic when producing aldoses from ketoses.
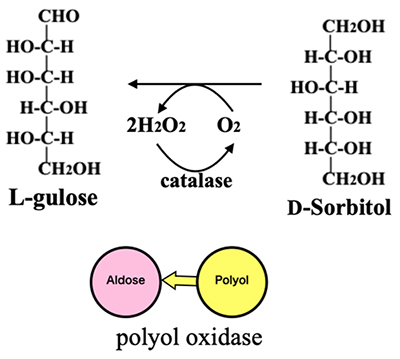
Besides isomerization, there is another possible way to produce aldose from ketose: the way that converts ketose to polyol and then oxidizes its terminal to produce aldose. An enzyme that facilitates this reaction is polyol oxidase, which can convert all polyols to aldoses in the presence of oxygen and catalase (Fig. 14). In fact, a polyol oxidase produced by a mold converts D-sorbitol to L-gulose in ~95% yield11. Talose and idose are difficult to produce using isomerase but will be obtained in high yield from talitol and iditol, respectively, if an appropriate polyol oxidase is available. Therefore, we started to rearrange the hexoses and hexitols in the original version of the Izumoring to develop the revised version of the Izumoring (Izumoring Ⅱ), which is based on the reaction pathway catalyzed by polyol oxidase.
The basis of the original version of the Izumoring was the ring arrangement of ketoses and hexitols. In the Izumoring Ⅱ, this basic structure is the same, but the ring at the center consists of only eight ketoses, which are surrounded by hexitols, and further by aldoses. Although the arrangement of the monosaccharides is changed in the Izumoring Ⅱ, the basic enzymatic reactions between the monosaccharides are identical to those of the original Izumoring version.
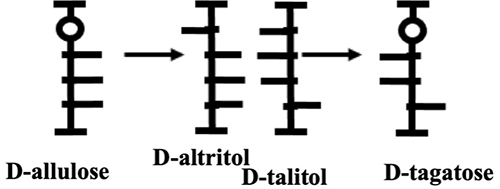
The original Izumoring schematic only listed the names of sugars, but we thought that providing molecular structures as well would enhance understanding and make the schematic easier to use. However, using the original Fischer projection formula would complicate the map. We therefore devised a space-saving notation and named it the Izumofleet formula after our collaborator, Professor George Fleet of Oxford University. For example, the conversion of D-allulose → D-altritol (D-talitol) → D-tagatose is depicted by Fischer projections as follows.
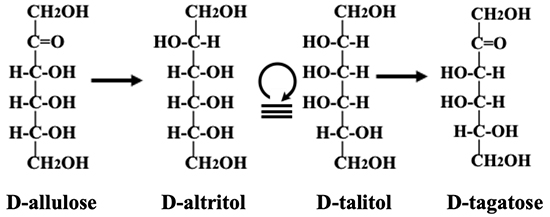
As shown in the figure (Fig. 15), using the Fischer projection formula makes the entire map complicated and difficult to understand. The Izumofleet formula is expressed according to the following rule and is simple and easy to understand (Fig. 16).
The reaction pathway in Fig. 15 can be described in terms of a Izumofleet formula as shown in the figure below (Fig. 17).
Finally, Fig. 18 shows the new strategy map of rare sugar production (the Izumoring Ⅱ), notated using Izumofleet formulas.
Both the original Izumoring (Izumoring Ⅰ) and Izumoring Ⅱ have the same basic cyclic arrangement of 34 hexoses interrelated by enzymatic reactions: a ring composed of eight ketoses, surrounded by D- and L-aldoses in point-symmetric positions. The role as the strategy map for rare sugar production is also the same. However, the Izumoring Ⅱ shows the pathways of sequential conversion from ketoses in the center to the surrounding polyols and then to the most outer aldoses (indicated by blue lines). The conversion of ketoses to polyols is assumed to be hydrogenation catalyzed by Raney nickel, and the conversion of polyols to aldoses is assumed to be oxidation with polyol oxidase; we expect that this pathway will efficiently produce thermodynamically unstable sugars such as idose. The red lines between ketoses and aldoses are the isomerization reaction by isomerases (not via polyols), and these pathways can be used to produce relatively thermodynamically stable aldoses from ketoses (e.g., allulose → allose). We first started the development of the Izumoring Ⅰ with relating all hexoses by enzymatic reactions using D-glucose as the starting material; on the other hand, the Izumoring Ⅱ is a strategy map for producing all hexoses, focusing on conversion from ketose.
The Izumoring Ⅱ is an evolved version of the Izumoring Ⅰ. The appropriate one can be selected and used effectively depending on the situation. For example, the Izumoring Ⅰ should be chosen when the raw material is an easily available aldose such as D-glucose or a polyol, whereas the Izumoring Ⅱ should be used when the raw material is a ketose.
From the next article, we will introduce the properties of each rare sugar, the enzymes used for rare sugar production, and the current state of the industry-academia-government collaboration for developing applications of rare sugars.