
Daiki Yamamoto
Dr. Daiki Yamamoto received his Ph. D. in science in March 2022 from the Faculty of Science and Technology, Keio University. He is interested in the various functions of glycans in cancer cells.
He has been engaged in drug discovery research as a pharmaceutical scientist at a pharmaceutical company since April 2023.

Toshinori Sato
Current position: Professor at the Department of Biosciences & Informatics, Faculty of Science and Technology, Keio University
Academic background: Dr. Sato completed his master’s course at the Kyushu University Graduate School of Engineering Sciences in March 1983, withdrew from his doctoral course in September 1983, and obtained a doctoral degree in engineering from Kyoto University in 1990. Work career: He was appointed Assistant Professor at the Nagasaki University Faculty of Engineering in October 1983, Assistant Professor at the Kyoto University Faculty of Engineering in October 1990, Associate Professor at the Tokyo Institute of Technology Faculty of Life Science and Technology in April 1992, and Associate Professor at Keio University Faculty of Science and Technology in April 2000. He has held his current position since April 2002.
Dr. Sato was a recipient of the CSJ Award for Young Chemists in 1993, and the Bio Business Competition Japan The Takeda Foundation Award in 2010.
Research interests: Dr. Sato’s research interests include glycomics using saccharide primers, polysaccharide-based drug delivery systems, lipid raft formation and recognition functional analysis using biomembrane models, and search and functional analysis of glycan-related peptides using a phage-display method.
Prostate cancer (PCa) is the most common malignant tumor in men. As the number of patients increases, the number of patients with a highly malignant treatment-resistant form of prostate cancer is increasing, which is a problem. Therefore, there is a need to develop diagnostic and therapeutic methods for malignant prostate cancer. Prostate-specific antigen tests are currently used to diagnose PCa, but they have a high rate of false positives that often require the use of invasive diagnostic interventions. Therefore, more accurate diagnostic methods are needed. The authors elucidated the expression and function of O-glycans in PCa cells that have become malignant under hormone deprivation and hypoxic conditions. In order to understand the glycan structures expressed in cells, detection with antibodies and lectins, enzymatic and chemical excision, and analysis using mass spectrometry are being used. In addition, the saccharide primer method has been developed to analyze glycans expressed in cultured cells. In this article, we will introduce the results of O-glycan analysis using the saccharide primer method in prostate cancer cells that became malignant after hormone removal or in a hypoxic environment, as well as functional analysis of these glycans.
Glycans are involved in various biological functions such as cell proliferation and differentiation, viral infection, and cell-cell adhesion1. Therefore, clarification of the structure and function of glycans is important for understanding biological phenomena. Cancer cells express different sugar chains from normal cells due to abnormal expression or changes in the activity of glycosyltransferases, so they are used clinically as tumor markers2. One of the problems in cancer treatment is metastasis. Metastasis is a major cause of poor prognosis in patients, but the detailed mechanisms remain largely unknown. It has been reported that glycans and glycosyltransferases are involved in metastasis. Therefore, to understand the mechanisms of metastasis, it is important to elucidate the functions of glycans and necessary to comprehensively analyze the glycans expressed in cells.
The saccharide primer method has been established to synthesize glycan libraries and to conveniently analyze glycans expressed in cells3-11. The method is described in detail in a previous article12. This method is used to construct glycan libraries by changing the combination of saccharide primers and cells. Subsequently, the method has also been used for the analysis of glycan synthesis pathways in cells with the aim of utilizing it for comparative glycomics. We hope that analyzing the sugar chain synthesis pathways expressed in various cells will be useful in clarifying the functions of sugar chains in cellular phenotypes. Saccharide primers such as Lac-C12, GlcNAc-C12, GalNAc-Ser-C12, GalNAc-Thr-C12, and Xyl-Ser-C12 have been developed to obtain ganglio-, globo-, neolacto-type glycans, mucin-type O-glycans, and glycosaminoglycans, respectively. When administered to cells, saccharide primers that mimic the precursor structure for glycan biosynthesis are taken up and glycosylated by glycosyltransferases. Glycosylated products are secreted into the medium. By analyzing glycosylated products using liquid chromatography-mass spectrometry (LC-MS), it is possible to deduce glycans expressed in cells (Fig. 1A). In a study using prostate cancer cells, O-glycan expression was analyzed after adding GalNAc-Thr-C12 primer to the cells (Fig. 1B).
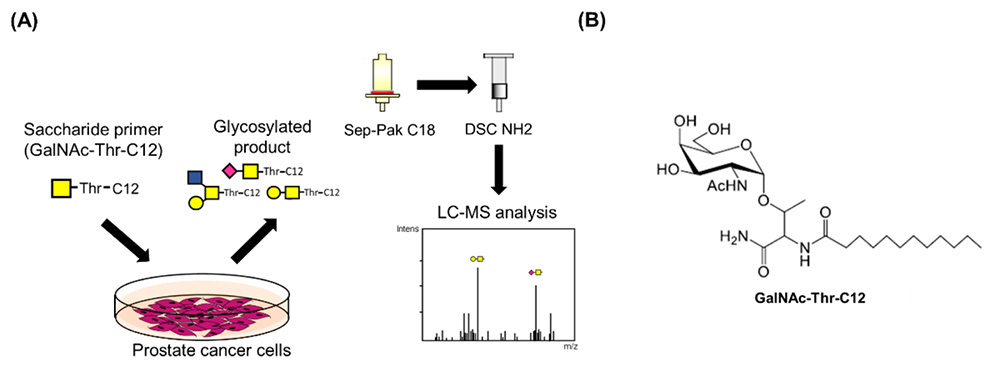
PCa is the most frequently diagnosed malignant cancer in men13. The 5-year survival rate for PCa patients is relatively high compared to other types of cancer, at over 90%14. However, the problem is that the number of patients with highly malignant treatment-resistant forms of prostate cancer is increasing15.
Prostate cancer cells proliferate when the male hormone androgen binds to the androgen receptor (AR). Therefore, androgen deprivation therapy targeting the AR-mediated signaling pathway is used to inhibit cancer cell growth16. In many cases, continued hormone therapy results in the appearance of prostate cancer cells that proliferate in an androgen-independent manner. Such prostate cancer cells are called castration-resistant prostate cancer cells (CRPC) and are highly malignant17. Therefore, research on new diagnostic markers and drug targets for CRPC is urgently needed.
Prostate-specific antigen (PSA) testing is commonly used for the diagnosis of PCa. Since a high blood level of PSA is often observed in benign prostatic hyperplasia and prostatitis, false positives occur at a high rate. Thus, invasive diagnostic methods such as prostate palpation and prostate needle biopsy are required after the PSA test18. Therefore, a less invasive and more accurate diagnostic marker for detection of CRPC is needed. Glycans that are characteristically expressed in CRPC cells are expected to contribute to the research and development of novel markers for this purpose. However, the expression and function of O-glycans in CRPC is unknown.
The tumor microenvironment (TME) contains a heterogeneous population of normal, cancer, and immune cells, where insufficient angiogenesis or drug resistance can occur19,20. Hypoxia is characteristic of the microenvironment of most solid tumors21. Oxygen does not diffuse to a region more than 100 μm away from blood vessels in the tumor tissue, and tumor cells in such an environment are subject to extreme hypoxia. However, the expression of hypoxia-inducible factor α (HIF-1α) allows cancer cells to survive under hypoxic conditions. Furthermore, angiogenesis, metastasis, chemoresistance, radioresistance, apoptosis resistance, etc. are promoted in cancer cells under hypoxia22,23. PCa tissue also has hypoxic regions24, and hypoxia is known to be a major factor in the malignant transformation of prostate cancer25. Because currently no treatments for PCa under hypoxia are effective26, there is an urgent need for elucidating the mechanisms by which prostate cancer metastasizes and acquires drug resistance in a hypoxic environment. The O-glycans in several cancers under hypoxia have been investigated. For example, the expression of Tn antigens is increased in breast cancer cells under hypoxia compared to that under normoxia27. However, the expression and function of O-glycans in PCa cells under hypoxic condition have not yet been investigated.
The CRPC cell lines were established by Professor Mototsugu Oya of Keio University School of Medicine and his colleagues28. LNKO6, 18, and 24 cells were established by subculturing LNCaP cells in androgen-free medium for 6, 18, and 24 months, respectively. To investigate the malignancy of CRPC cells employed in this study, cell proliferation and migration ability were evaluated. The CRPC cells showed increased proliferation and migration ability in androgen-free medium compared to LNCaP cells. These results suggested that CRPC cell lines have castration resistance and their malignancy increased depending on the androgen-deprivation period.
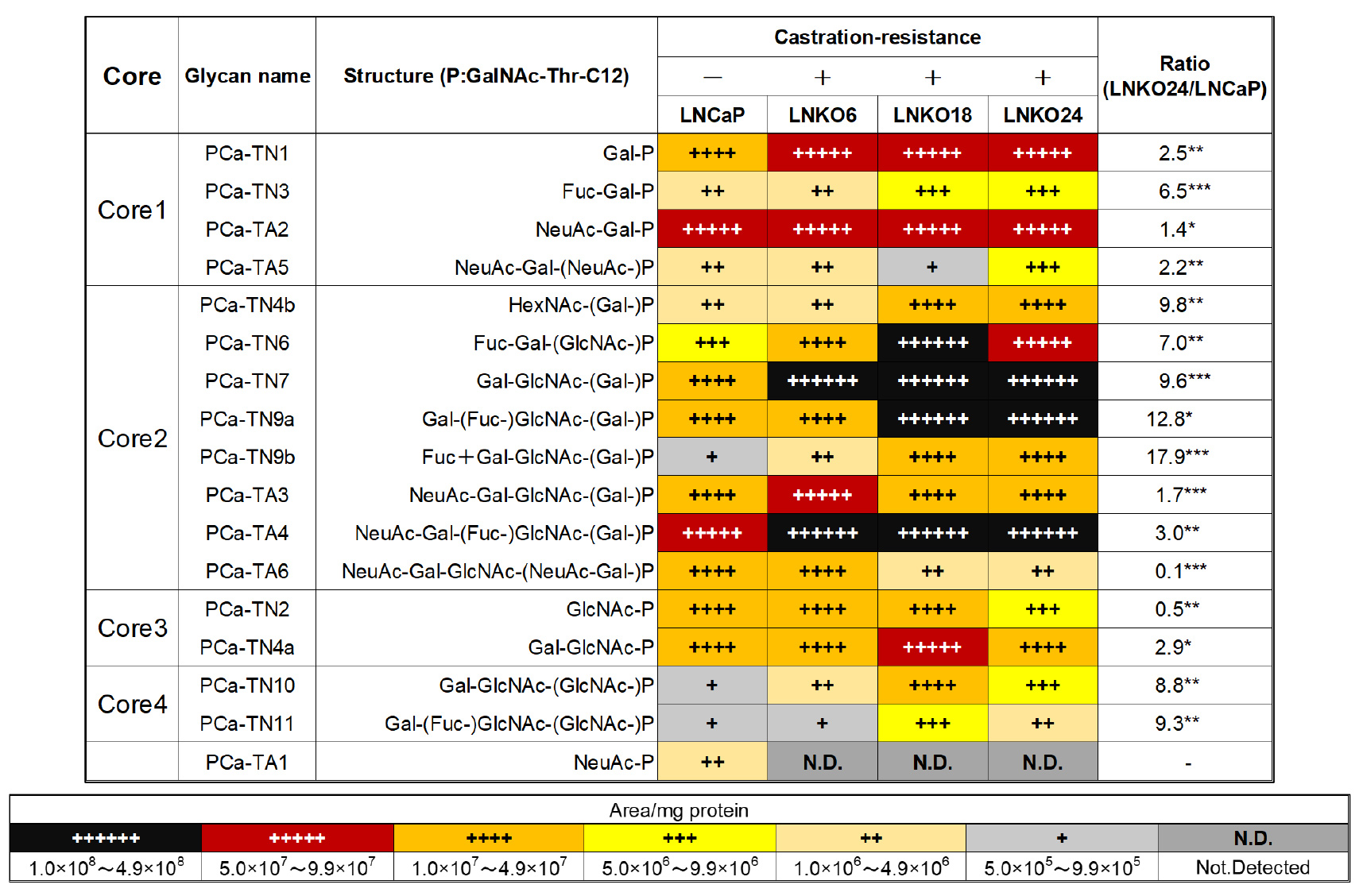
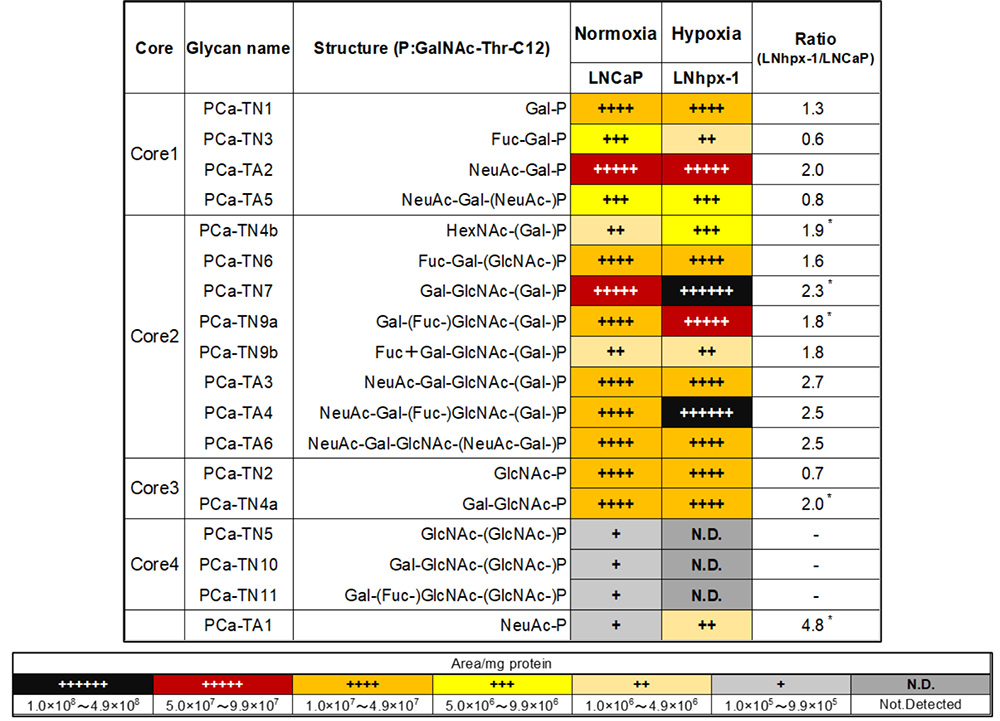
By adding the saccharide primer GalNAc-Thr-C12 to CRPC cells, O-glycan type glycosylated products were obtained. Sequences and amounts of glycosylated products were analyzed using multiple reaction monitoring (MRM) modes of LC-MS. As shown in Table 1, the expression levels of core 2/4-type O-glycans differed between LNCaP and CRPC cells. In particular, the expression of core 2-type O-glycans such as Lex, sLex antigen (PCa-TN9a, TA4) and core 4-type O-glycans (PCa-TN10, TN11) was significantly increased in CRPC cells. However, sTn antigen (PCa-TA1), a tumor marker for many cancers, was not detected in these cells29. Since biosynthesis of O-glycans is competitive between core structures, it was suggested that the biosynthesis of core 2/4-type O-glycans competes with that of sTn antigen in CRPC cells. Although we have performed glycan analysis in many cell lines, PCa cells were the first cells to contain detectable core 4-type O-glycans. Therefore, we were interested in the function of core 4-type O-glycans.
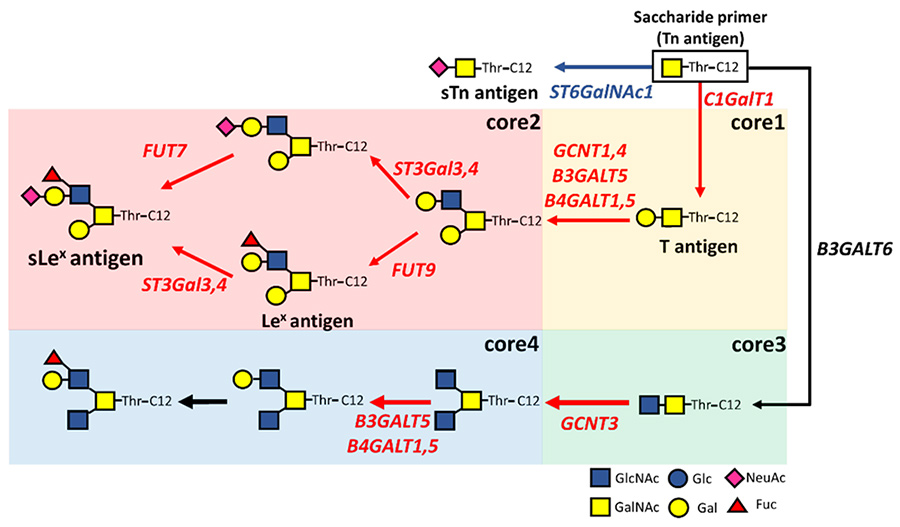
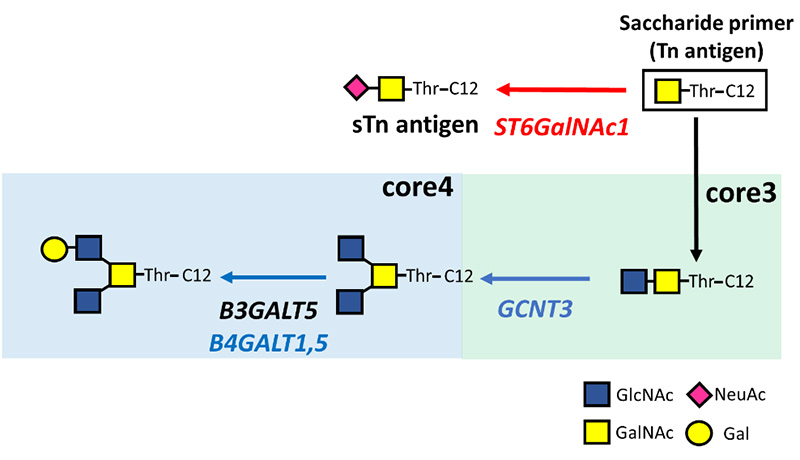
The expression of glycosyltransferase genes in CRPC cells related to the biosynthesis of O-glycans was analyzed by real-time RT-PCR. In CRPC cells, in addition to the expression of glycosyltransferase genes of core 1/2-type O-glycans, expression of GCNT3 involved in the biosynthesis of a core 4-type O-glycans was upregulated relative to its level in LNCaP cells (Fig. 2). In contrast, the sTn antigen synthase gene ST6GalNAc1 was significantly downregulated in CRPC cells. It was inferred that androgen deprivation controls the expression of glucosyltransferase genes, and promotes the core 4-type O-glycan biosynthesis pathways.
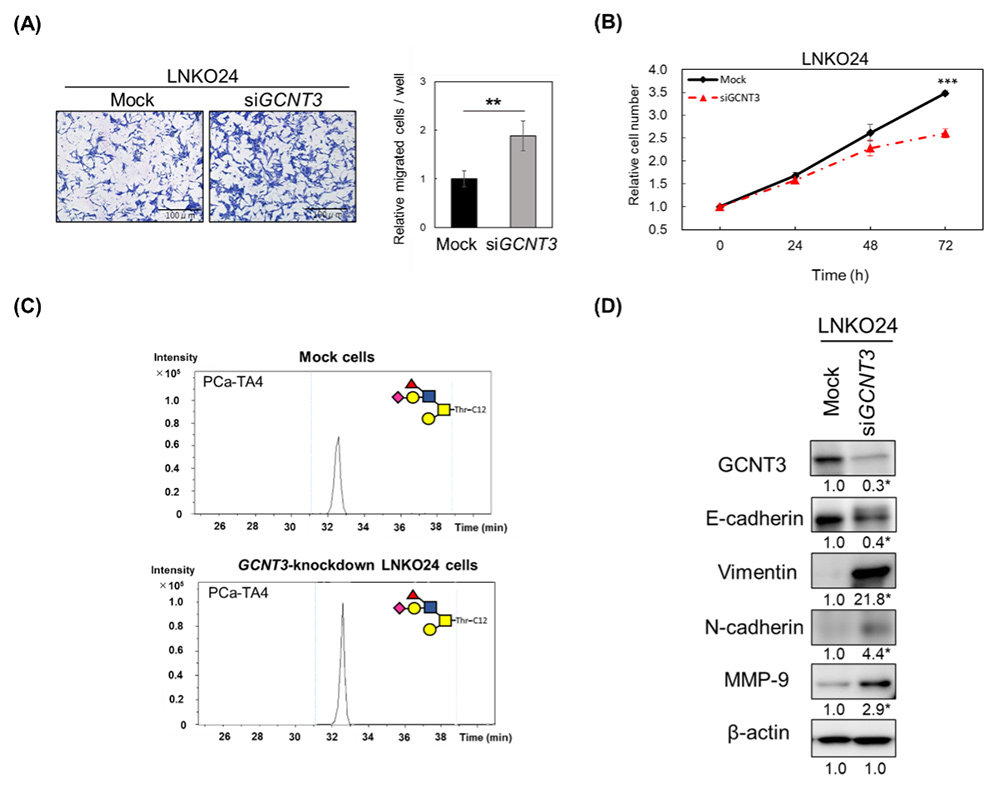
Since the expression of core 4 type O-glycan and its synthase gene GCNT3 was characteristically increased in CRPC cells, we analyzed the function of GCNT3 in CRPC cells. To this end, we performed knockdown of GCNT3 in LNKO24 cells using siRNA, and evaluated the cell proliferation and migration abilities of GCNT3 knockdown cells. GCNT3 knockdown significantly increased LNKO24 cell migration (Fig. 3A), but significantly decreased LNKO24 cell proliferation (Fig. 3B). Glycan expression analysis showed increased expression of sLex antigen in GCNT3-knockdown LNKO24 cells (Fig. 3C). It has been reported that sLex antigen expression is related to epithelial-to-mesenchymal transition (EMT)30. Thus, we evaluated the expression of EMT markers31 such as E-cadherin, vimentin, N-cadherin, and matrix metalloproteinase-9 (MMP-9). The results showed decreased expression of epithelial markers such as E-cadherin and increased expression of mesenchymal markers such as vimentin, N-cadherin, and MMP-9 in GCNT3-knockdown LNKO24 cells, compared to mock cells (Fig. 3D). Expression of core 4 type O-glycans suppressed cell migration and EMT, and promoted cell proliferation in the TME. These results suggest that core 4 type O-glycans play a role in adaptation to androgen-deprived environments32.
LNhpx-1 cells were established by culturing LNCaP cells at 37 °C in a humidified 5% CO2, 1% O2, and 94% N2 atmosphere for 2 months. Although the migratory ability of LNhpx-1 cells was higher than that of LNCaP cells, the proliferation ability of LNhpx-1 cells was equivalent to that of LNCaP cells under normoxia.
O-glycans expressed in LNhpx-1 cells under hypoxia were analyzed using the saccharide primer GalNAc-Thr-C12. As shown in Table 2, the expression of the sTn antigen (PCa-TA1) in LNhpx-1 cells was significantly increased compared to that in LNCaP cells. In contrast, core 4-type O-glycans (PCa-TN5, TN10, and TN11) were decreased below the detection limit in the LNhpx-1 cells. These results indicate that PCa cells under hypoxia showed distinctive O-glycan expression profiles.
The expression of glycosyltransferase genes in LNhpx-1 cells under hypoxic conditions was analyzed. The sTn antigen synthase gene ST6GalNAc1 was significantly upregulated in LNhpx-1 cells compared to LNCaP cells under normoxia (Fig. 4). In contrast, the expression of GCNT3 was significantly downregulated in LNhpx-1 cells. Analyzing O-glycans and glycosyltransferase genes revealed that the biosynthesis pathway of sTn antigen was activated in response to the hypoxic environment.
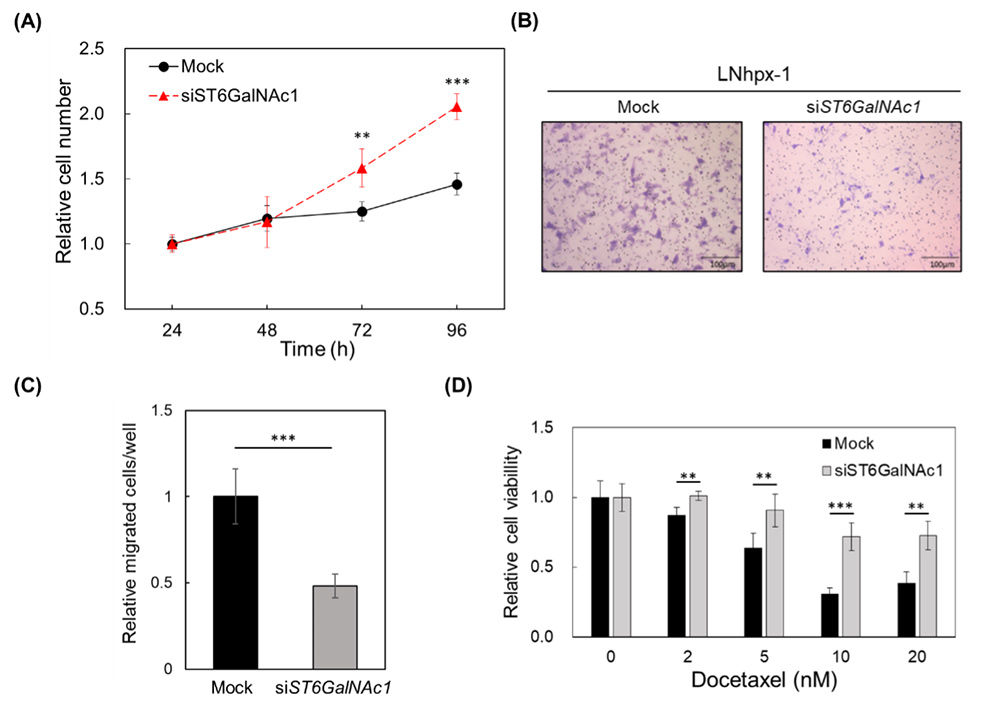
To investigate the functions of ST6GalNAc1 in LNhpx-1 cells under hypoxia, ST6GalNAc1 was knocked down using siRNA. Cell proliferation was significantly increased in ST6GalNAc1-knockdown cells (Fig. 5A), whereas cell migration was significantly decreased (Fig. 5B, C). It has been known that PCa cells under hypoxia are docetaxel resistant25. Therefore, the contribution of sTn antigen and ST6GalNAc1 to docetaxel resistance of LNhpx-1 cells in a hypoxic environment was investigated. ST6GalNAc1-knockdown cells showed higher resistance to docetaxel treatment (Fig. 5D). These results indicate that ST6GalNAc1 promotes cell migration while suppressing cell proliferation and drug resistance in LNhpx-1 cells under hypoxia.
Cancer cells do not proliferate while migrating, whereas they stop migrating while proliferating33. The phenomenon in which either migration or proliferation is promoted is called “migration-proliferation dichotomy”34. It has been reported that cell migration-proliferation dichotomy does not promote cell proliferation during metastasis, so metastatic cancer cells are not affected by anticancer drugs35. In our study, it was found that sTn antigen regulates migration–proliferation dichotomy under hypoxia36. In prostate cancer cells in a hypoxic environment, increased expression of sTn antigen promotes cell migration, and it is considered that they have acquired the ability to escape from the hypoxic environment.
We analyzed the O-glycans in prostate cancer cells using the saccharide primer method, which allows convenient glycan analysis. We focused on mucin-type O-glycans in malignant PCa cells, and comparatively analyzed their expression profiles. Distinctive O-glycans were observed in castration-resistant and hypoxic cells. These results indicate that the O-glycan biosynthetic pathway is regulated by tumor microenvironmental stresses such as androgen deprivation and hypoxia. Our results also suggest that altered O-glycan expression is one of the survival strategies of PCa cells.
Glycome analysis of disease-related cells using the saccharide primer method is an effective strategy for studying cellular regulatory function at the gene level. We hope that this study will lead to the elucidation of pathological mechanisms involving glycans in PCa and the development of novel diagnostic and therapeutic methods.