
Diana Egorova
Tokyo University of Agriculture and Technology, United Graduate School of Agricultural Science, Department of Applied Life Science.
I came to Japan in 2016 as a government-sponsored student and enrolled in the Department of Applied Biological Sciences, Faculty of Agriculture of Tokyo University of Agriculture and Technology. Upon graduating from the bachelors program, I proceeded to the master's program, and then the doctoral program in the same university. From my fourth year as an undergraduate, I became one of the first students in Prof. Shinji Miyata's laboratory and began researching the extracellular matrix of the brain. Among my research on extracellular matrices, I am particularly focused on hyaluronic acid, and have presented my findings at both domestic and international conferences.

Shinji Miyata
Associate Professor at Faculty of Agriculture, Tokyo University of Agriculture and Technology.
Dr. Shinji Miyata received his Ph.D. from Nagoya University in 2006 under the mentorship of Prof. Ken Kitajima, specializing in sialic acid research. He did postdoctoral research at UCSD under the direction of Prof. Victor D. Vacquier. In 2007, he initiated glycosaminoglycan research as a postdoctoral researcher under the supervision of Prof. Hiroshi Kitagawa at Kobe Pharmaceutical University. Following this, he served as an Assistant Professor at Nagoya University from 2013 to 2019. In 2019, he established his laboratory at Tokyo University of Agriculture and Technology, focusing on hyaluronan-proteoglycan complexes in the nervous system.
Hyaluronan (HA) is a central component of the extracellular matrix (ECM) in the central nervous system. Brain HA exists in two distinct forms of ECM: the diffuse ECM, which is soluble in saline and detergents, and the condensed ECM, which forms aggregates, such as perineuronal nets. Although the physiological functions of HA significantly differ depending on its size, size differences in HA have not yet been examined in the two ECM types. Recently, we established a simple method to simultaneously assess the molecular weight (MW) of HA in multiple crude biological samples. HA was purified through single-step precipitation from tissue extracts using biotinylated HA-binding protein and streptavidin-coupled magnetic beads, followed by separation on gel electrophoresis. By applying this method to HA in the mouse brain, we revealed that the condensed ECM contained higher MW HA than the diffuse ECM. Higher MW HA and lower MW HA exhibited different spatial distributions: the former was confined to perineuronal nets, while the latter was widely present throughout the brain. Furthermore, the limited degradation of HA showed that higher MW HA was required to form an insoluble HA-aggrecan complex. The present study demonstrated that the MW of HA in the brain strongly correlates with the localization and solubility of the ECM it forms.
Hyaluronan (HA), a central component of the extracellular matrix (ECM), is composed of repeating disaccharide units of glucuronic acid and N-acetylglucosamine. The molecular weights (MW) of HA range widely and may reach as high as several million Da1,2 . Recent studies revealed that HA regulates a series of biological reactions depending on its MW3-6.
The brain ECM consists of HA and a family of HA-binding chondroitin sulfate proteoglycans (CSPGs) such as aggrecan, versican, neurocan, and brevican7,8. These CSPGs share multiple structural domains, including an N-terminal globular domain that binds to HA and a C-terminal globular domain that attaches to tenascin-R. HA in the brain forms two distinct types of ECM: diffuse and condensed ECM9,10. The diffuse ECM comprises loose saline- and detergent-soluble structures found throughout the brain parenchyma. The condensed ECM forms aggregates that may only be extracted from tissues after denaturation by urea. Perineuronal nets (PNNs) around a subpopulation of neurons are a prominent example of the condensed ECM. In the rodent brain, the diffuse ECM is predominant during early development, and PNNs then emerge approximately one month after birth11,12.
We previously reported that 30-40% of all HA in the adult mouse brain, was present in the diffuse ECM and 60-70% in the condensed ECM13. However, the molecular mechanisms that give rise to these two ECM types remain unclear. Specifically, potential differences in the size of HA in these ECM have not yet been examined. In this article, we expound upon a novel technique recently devised to assess the MW of HA in multiple crude biological samples14.
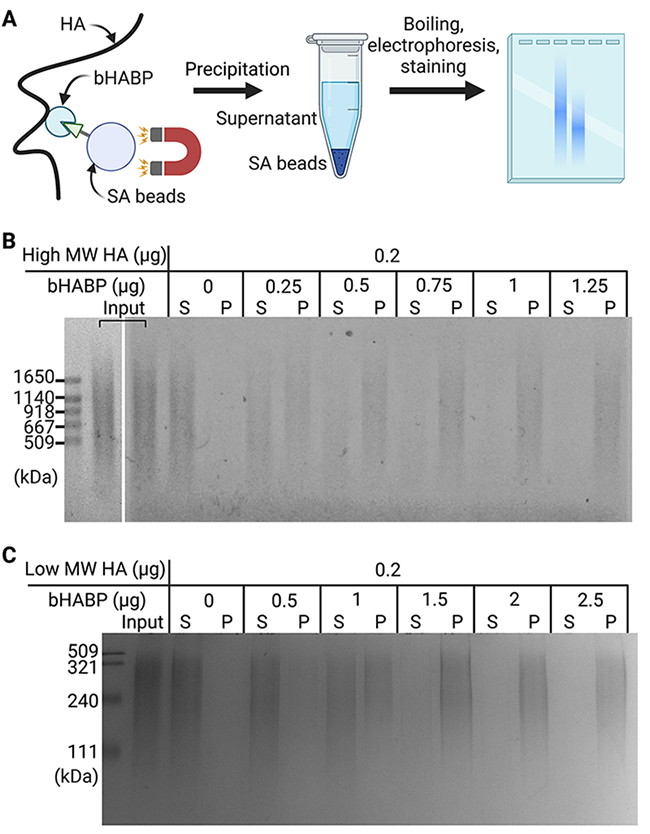
First, we examined the amount of biotinylated HA-binding protein (bHABP) required to precipitate 0.2 μg of pure high MW HA (600 to 1120 kDa). HA in solution was incubated with bHABP and then precipitated with streptavidin-coupled magnetic (SA) beads (Figure 1A). After heat denaturation, released HA was separated by agarose gel electrophoresis and detected with Stains-All. HA was precipitated depending on the amount of bHABP added, and 1 μg of bHABP was sufficient to precipitate 0.2 μg of high MW HA. We noted that higher MW HA was preferentially precipitated over lower MW HA when insufficient amounts of bHABP were used (Figure 1B). Under the same experimental conditions, the precipitation of low MW HA (100 to 300 kDa) required a ∼2-fold higher amount of bHABP than that of high MW HA (Figure 1C). These results demonstrated the ability of our method to capture and detect HA with a broad range of MW. However, it is important to initially quantify the content of HA in samples and use sufficient amounts of bHABP because an insufficient quantity of bHABP leads to the underestimation of low MW HA.
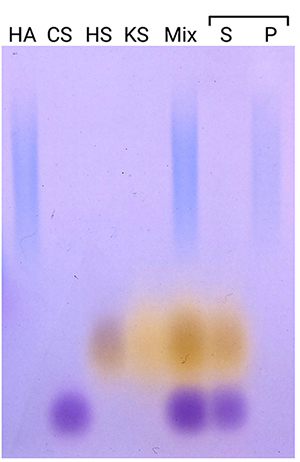
To examine the applicability of our method to samples containing not only HA, but also several glycosaminoglycans, we prepared a mixture of four glycosaminoglycans: HA, chondroitin sulfate A, heparan sulfate, and keratan sulfate (Figure 2). Only HA was precipitated, while the other glycosaminoglycans remained in the supernatant, demonstrating the specificity of the method to separate HA.
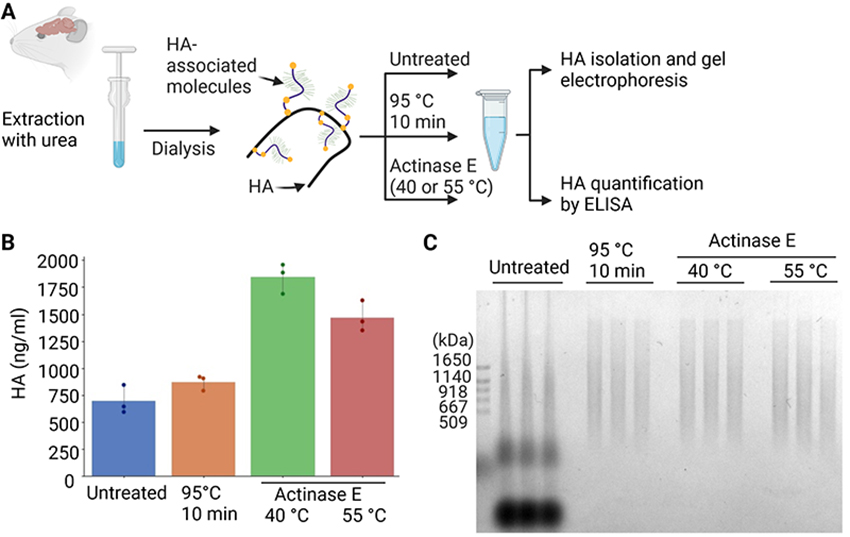
Next, we aimed to establish a method to isolate HA from crude brain extracts without preparing glycosaminoglycans fractions. Mouse brains were homogenized in phosphate-buffered saline (PBS) containing 6 M urea, and the resultant crude extracts were dialyzed against PBS to deplete urea (Figure 3A). Crude extracts were untreated, heat denatured at 95°C, or extensively digested by actinase E, a bacterial protease displaying a wide range of substrate specificities. The quantification of HA by an enzyme-linked immunosorbent assay (ELISA) indicated that actinase E digestion at 40°C yielded the highest amount of HA among the various conditions examined (Figure 3B). Precipitation from untreated extracts was unsuitable for the MW analysis because multiple components were detected, possibly due to the association of HA with proteins (Figure 3C). In contrast, the heat denaturation or actinase E digestion of the brain extract resulted in the clear separation of HA, allowing for an analysis of the MW of HA on gel electrophoresis. In subsequent experiments, we digested tissue samples with actinase E at 40°C before HA isolation.
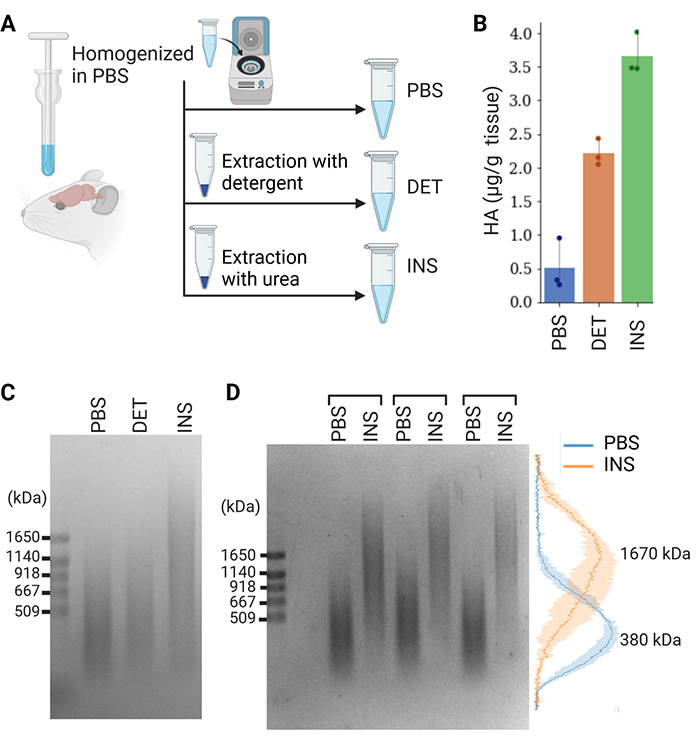
We investigated whether the MW of HA in the diffused and condensed ECM differed by using our method. Previous studies demonstrated the recovery of the diffuse ECM in PBS-soluble and detergent-soluble fractions and the condensed ECM in an insoluble fraction9,15. Therefore, we sequentially extracted PBS-soluble, detergent-soluble, and insoluble components from the adult mouse brain (Figure 4A). Quantification by ELISA indicated that the PBS-soluble, detergent-soluble, and insoluble fractions contained 8, 35, and 57% of total HA, which was consistent with our previous findings (Figure 4B)10,13. We found that the MW of HA in the insoluble fraction was markedly higher than that in the PBS-soluble and detergent-soluble fractions (Figure 4C). Normalized intensity profiles confirmed that the peaks of the size distributions of PBS-soluble and insoluble HA were 380 and 1670 kDa, respectively (Figure 4D).
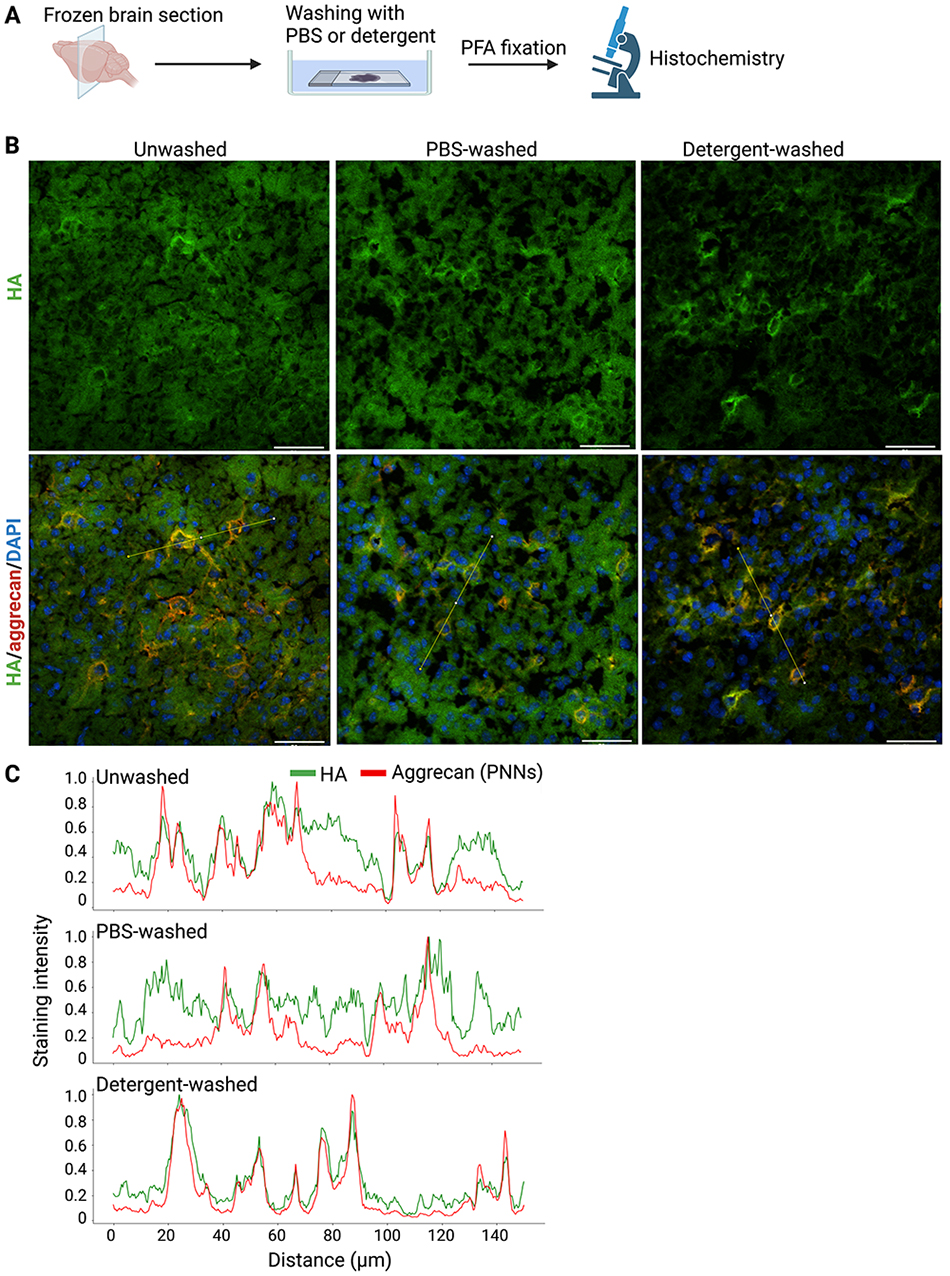
To examine the spatial distribution of higher MW HA recovered in the insoluble fraction and lower MW HA in the PBS- and detergent-soluble fractions, we prepared fresh frozen sections of the mouse brain. After washing with PBS or detergent, we fixed brain sections with paraformaldehyde PFA and visualized HA with bHABP (Figure 5A). HA was distributed throughout the mouse cerebral cortex in unwashed or PBS-washed sections (Figure 5B). Strong HA staining in the pericellular region corresponded to PNNs because of its co-localization with aggrecan, a major CSPG in PNNs. A significant percentage of HA was also present outside PNNs corresponding to neuropil (Figure 5B, C). After washing with detergent, HA present in PNNs remained, whereas HA in the non-PNN region was washed away from brain sections (Figure 5B, C). These results showed that the higher MW HA in the insoluble fraction and lower MW HA in the PBS- and detergent-soluble fractions exhibited different spatial distributions: the former was confined to PNNs, while the latter was widely present in the non-PNN regions.
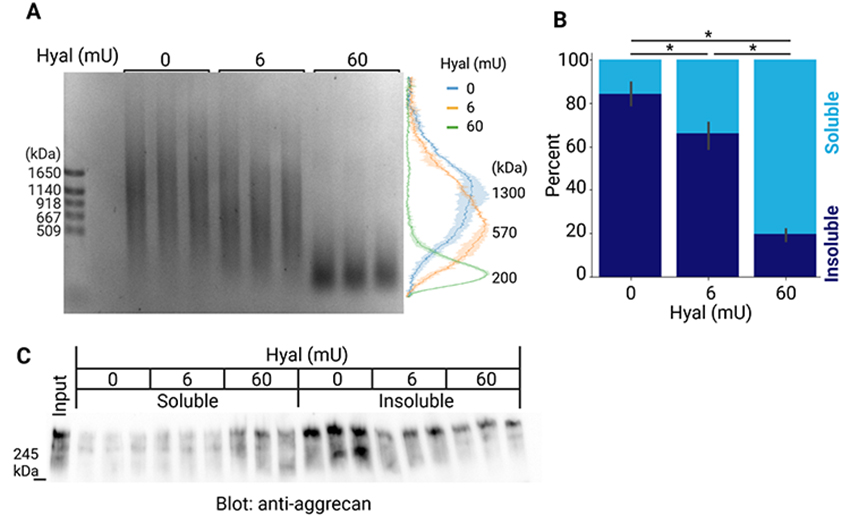
To investigate whether higher MW HA is necessary for the formation of aggregates of PNNs, we partially digested HA in the insoluble fraction of the mouse brain with hyaluronidase. The peak of the size distribution of HA in the insoluble fraction was 1300 kDa, and shifted to 570 and 200 kDa after digestion with 6 and 60 milliunits of hyaluronidase, respectively (Figure 6A). After hyaluronidase digestion, we separated soluble and insoluble components by centrifugation. The quantification of HA indicated that its solubility was significantly increased by the limited degradation of HA (Figure 6B). This suggests that a longer HA appears to be required to maintain dense aggregates of PNNs. We also examined whether the limited digestion of HA increased the solubility of molecules that associate with HA, such as aggrecan. The immunoblot detection of aggrecan revealed that its solubility also significantly increased after the limited digestion of HA (Figure 6C).
Although HA is naturally polydisperse in all organisms, it is currently unknown whether HA of different sizes exhibits a differential distribution within tissues or is uniformly present throughout tissues. The tissue distribution of HA has been examined by utilizing HA-binding protein domains, such as bHABP, as probes16. However, these probes are incapable of distinguishing the MW of HA, rendering it infeasible to locate the HA of a particular MW. The present study enabled us to examine the distribution of lower and higher MW HA in the mouse brain based on their distinct biochemical characteristics, with the former being extracted by detergents, while the latter was not. We found that higher MW HA was present in PNNs, while lower MW HA was widely distributed throughout the neuropil in the cerebral cortex. This approach may be extended to tissues beyond the mouse brain, and future investigations may reveal the localization of HA with different sizes in tissues of various species.
Emerging evidence has indicated that the formation of PNNs inhibits brain plasticity and promotes memory retention in the adult brain8,17. The enzymatic removal of PNNs facilitates the erasure of fear memories and prevents their consolidation, suggesting that they are necessary for the long-term protection of memories18,19. Mechanistically, aggregates of HA and CSPGs may act as physical barriers that stabilize pre-existing synapses and prevent the formation of new synaptic connections20-22. In the present study, the limited degradation of high MW HA suggested that HA longer than 1000 kDa may be required to maintain insoluble HA-aggrecan complexes in PNN, indicating the involvement of long HA in memory maintenance. We previously reported that the MW of HA in the brain decreased with aging, thereby increasing the solubility of the HA-aggrecan complex13. Further studies are needed to examine changes in the MW of HA under neurodegenerative conditions and to gain a more comprehensive understanding of the role of HA in these diseases.