
Toshiki Nokami
Professor, Tottori University
He obtained his PhD from Kyoto University under the supervision of Professor Jun-ichi Yoshida in 2004. After 11 months of postdoctoral training at ETH Zurich under the supervision of Professor Peter H. Seeberger, he joined the group of Professor Jun-ichi Yoshida as an Assistant Professor in 2005. He was promoted to Lecturer in 2011, then he moved to Tottori University as an Associate Professor in 2012. He started his research group in 2019 and was promoted to Professor in the same year. His research interests include carbohydrates, organic electrochemistry, and ionic liquids.
Enzymatic methods and chemical methods are two major methodologies for the synthesis of oligosaccharides. The enzymatic method using glycosyl transferase and/or glycosidase is useful for synthesizing oligosaccharides without introduction of protecting groups of hydroxyl groups. By contrast, the chemical method is based on techniques of synthetic organic chemistry. Thus, it is necessary to protect and deprotect reactive functional groups; however, both natural and unnatural oligosaccharides can be prepared. To synthesize these oligosaccharides, a number of building blocks have been developed and a variety of activation methods have also been reported for each of the building blocks. In this article, the electrochemical glycosylation of thioglycosides, which are widely used for chemical synthesis of oligosaccharides, is introduced. Although thioglycosides are stable and easy to handle, they can be selectively activated under electrochemical conditions.
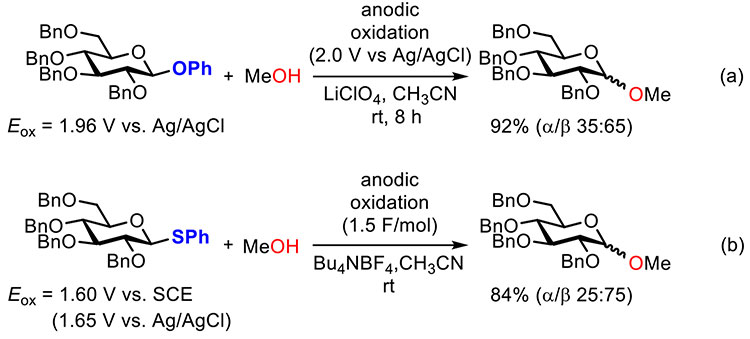
Electrochemical glycosylation, which is based on electrochemical activation of building blocks, was first reported by Noyori and Kurimoto (Figure 1a)1. Phenyl glycosides equipped with an electron-rich phenyloxy (PhO) group at the anomeric carbon were used as building blocks and electrochemically oxidized to form a glycosidic bond with methanol under the constant potential condition (2.0 V vs. Ag/AgCl). Electrochemical glycosylation using thioglycosides, which have lower oxidation potentials than phenyl glycosides, was also reported (Figure 1b)2,3. Thioglycosides equipped with a more electron-rich thiophenyl (PhS) group at the anomeric carbon were also used as building blocks for electrochemical glycosylation under the constant current condition (consumed electricity: 1.5 F/mol). Although the electrochemical method is useful to activate thioglycosides under mild reaction conditions, it had been used only for synthesis of short oligosaccharides up to trisaccharides4.
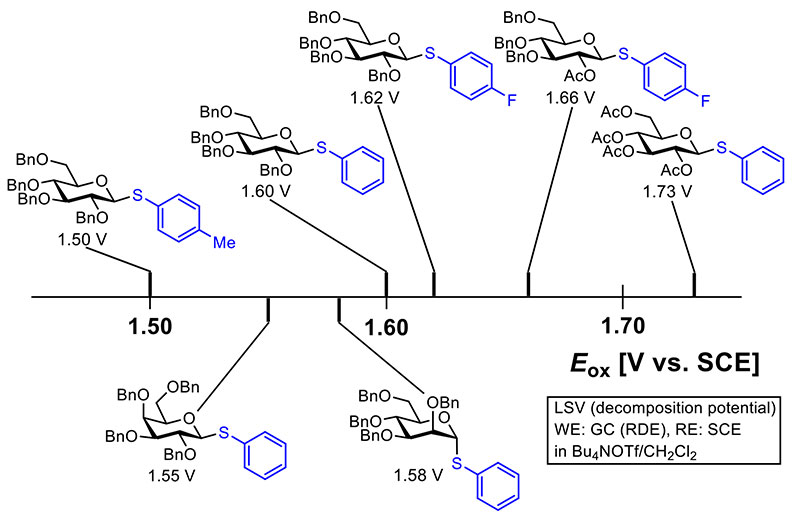
One of the benefits of electrochemical reactions of organic compounds is that the reactivity of the compounds can be evaluated by oxidation or reduction potentials quantitatively5. This is also true for electrochemical glycosylation. Of course, it is desirable that oxidation potentials are measured under the same conditions such as solvent, electrolyte, and reference electrode for comparison. The magnitude of oxidation potentials can be influenced by the conditions of measurement; however, we can directly compare oxidation potentials with different reference electrodes by correction. The oxidation potentials of some thioglycosides measured by using a rotating disk electrode (RDE) are shown in Figure 2. Oxidation potentials of thioglycosides are influenced by the electronic property of the substituent on the sulfur atom because the oxidation potential is the anodic potential necessary for the single electron oxidation of the sulfur atom. Therefore, a thioglucoside with an electron-rich substituent (Tolyl group = 4-methylphenyl) on the anomeric sulfur atom has lower oxidation potential (1.50 V vs. SCE) than that with phenyl group (1.60 V vs. SCE), and a thioglucoside with an electron poor substituent (4-FC6H4 = 4-fluorophenyl) has higher oxidation potential (1.62 V vs. SCE). Moreover, oxidation potentials depend on the protecting group of hydroxyl groups and the structure of monosaccharides. For example, electron withdrawing protection groups of hydroxyl groups such as the acetyl (Ac) group raise the oxidation potential of thioglycoside (1.66 V vs. SCE), and more acetyl groups influence oxidation potential more (1.73 V vs. SCE). Galactoside (1.55 V vs. SCE) and mannoside (1.58 V vs. SCE) have lower oxidation potentials than glucoside (1.60 V vs. SCE), even though they have the same anomeric substituent and protecting groups. Oxidation potentials of thioglycosides can be estimated by theoretical calculation as well. Difference in oxidation potentials of thioglycosides has been estimated by comparison of the difference in highest occupied molecular orbital (HOMO) energies obtained by using a density functional theory approach (B3LYP)6,7.
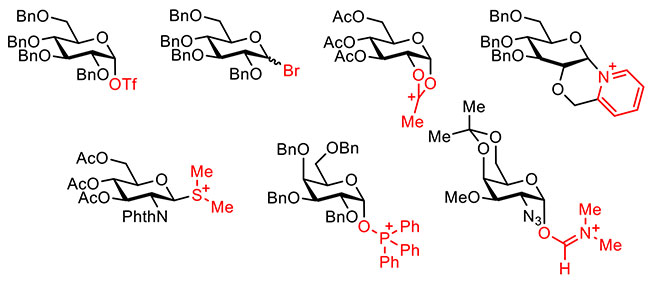
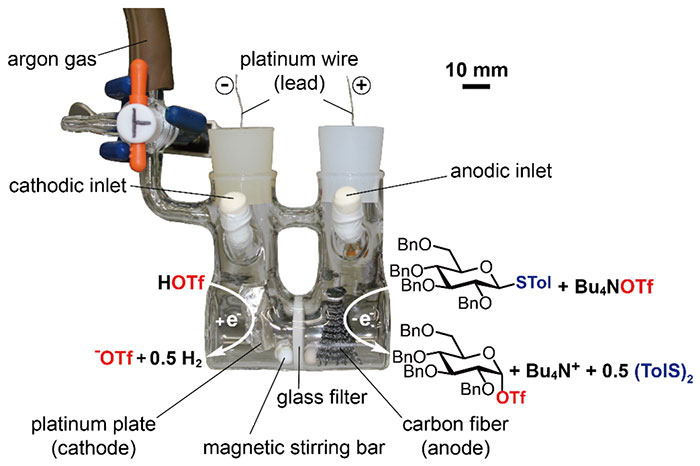
Advancement of chemical glycosylation technology has enabled spectroscopic observation of various glycosylation intermediates including glycosyl halides and triflates equipped with an anomeric halogen atom and triflate, respectively (Figure 3)8. Generation of a glycosyl cation intermediate has been proposed in electrochemical glycosylation; however, the reactive intermediate had never been confirmed by spectroscopic analysis. The author and co-workers tried to generate and accumulate the glycosylation intermediate using a divided electrolysis cell (Figure 4). Glycosyl triflates9,10,11,12 and glycosyl dioxalenium ions13 were generated and accumulated by anodic oxidation of thioglycoside in the presence of Bu4NOTf as electrolyte at low temperature. These intermediates have already been detected by low-temperature NMR measurement of anodic solution and their reaction with a sugar hydroxyl group formed glycosidic bonds.
Automated synthesis of an oligosaccharide base on solid-phase synthesis using polymer resin or porous material has already been reported; however, there were many drawbacks14. As shown in Table 1, solid-phase synthesis is not useful for large scale production, and excess amounts of reagents and solvents are necessary. Therefore, development of a method for automated synthesis of oligosaccharide based on solution-phase synthesis has been desired. Although we found that the electrochemical method was useful to generate highly reactive glycosylation intermediates, we were not aware of its application. Further information of their reactivity inspired us to apply electrochemical glycosylation to automated electrochemical solution-phase synthesis of oligosaccharides.
| Items | Solid-phase synthesis | Solution-phase synthesis | Checking of reaction progress | After cleavage from solid support | Without pretreatment | Amount of reagent | Large excess | Theoretical - small excess | Repeat of reaction | Easy (necessary) | Difficult (unnecessary) | Washing | Easy (necessary) | Difficult (unnecessary) | Scale up | Difficult | Easy |
|---|
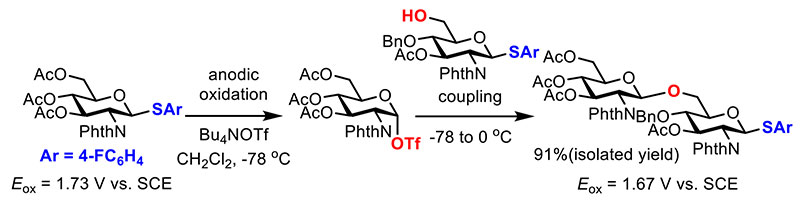
The most important step of automated electrochemical solution-phase synthesis of oligosaccharides is the reaction between the accumulated glycosyl triflate intermediate and building block (Figure 5)15. The building block in this process must be equipped with a protecting-group-free hydroxyl group and an anomeric leaving group which can be activated under electrochemical conditions. Possible side reaction is the reaction between the glycosyl triflate intermediate and the anomeric leaving group; however, introduction of an electron poor substituent (4-fluorophenyl group) prevented the side reaction, and the desired glycosylation proceeded selectively. Oxidation potential of disaccharide (1.67 V vs. SCE) was 0.06 V lower than that of monosaccharide building block (1.73 V vs. SCE). This fact suggested that electrochemical generation of the glycosyl triflate of longer oligosaccharides could be performed under similar electrochemical conditions.
To perform electrochemical generation of glycosyl triflate intermediate and its reaction with building blocks at raised temperature, the authors and co-workers built the automated electrochemical synthesizer by assembling commercially available instruments (Figure 6). In the first version of the synthesizer, the chiller, syringe pump, and power supply were controlled by a sequencer which was replaced by a personal computer. In the latest version of the synthesizer, all these instruments are put into a box equipped with a touch panel which displays all necessary parameters of the reaction.

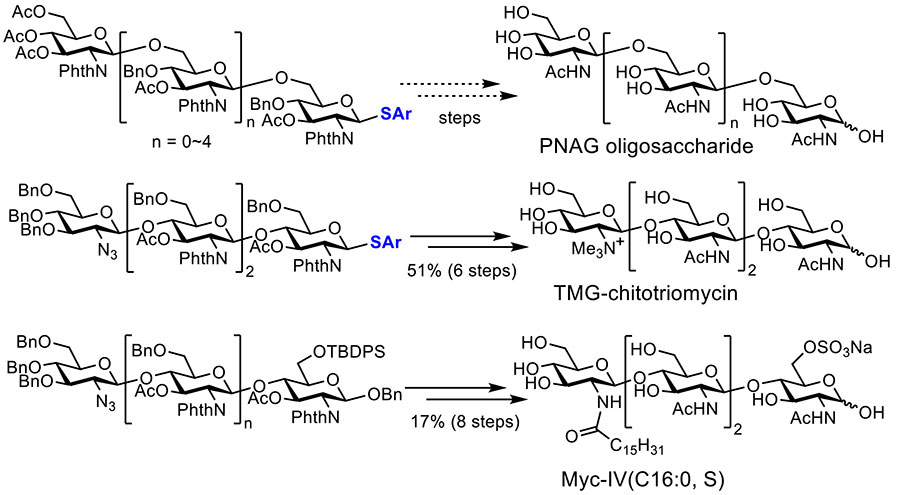
We have already synthesized several precursors of oligosaccharides such as the hexasaccharide of β-1,6-poly-N-acetylglucosamine (PNAG)15, tetrasaccharide of TMG-chitotriomycin6,16, tetrasaccharide of Myc-IV(C16:0, S)17, and hexasaccharide of cyclic β-glucan undecasaccharide18 (Figure 7). Some of these precursors have already been converted to the corresponding natural oligosaccharides. In the near future further optimization of building blocks and reaction conditions of electrochemical glycosylation may contribute to improvement of their yields and synthesis of structurally different oligosaccharides.
In this article, the pioneering works of electrochemical glycosylation and its applications ranging from generation of reactive glycosylation intermediates to automated electrochemical assembly of oligosaccharides have been introduced. Electrochemical glycosylation can be replaced by conventional chemical glycosylation because electrochemical glycosylation is one of the chemical methods used for glycosylation. But electrochemical glycosylation can be easily performed by the automated electrochemical synthesizer. Moreover, combining the synthesizer with analytical instruments and purification instruments will enable rational optimization of glycosylation by machine learning and complete automation from building blocks to pure oligosaccharides. Automated electrochemical assembly must be useful not only for synthesis of oligosaccharides but also other organic molecules. Therefore, we showed the great potential of automated electrochemical assembly by using it to synthesize complex oligosaccharides.