
Shoko Nishihara
Director, Glycan & Life System Integration Center (GaLSIC).
Professor, Department of Bioscience, Graduate School of Science and Engineering, Soka University.
She received her Ph.D. in 1982 from the Department of Chemistry, Graduate School of Science, the University of Tokyo. Then she worked at Keio University, the Jikei University School of Medicine, the University of North Carolina at Chapel Hill, and Mitsubishi Kasei Institute of Life Sciences, and has been at Soka University since 1991. During this time, she conducted research on Drosophila development and the Drosophila nervous system at University of North Carolina and Mitsubishi Kasei Institute of Life Sciences. After moving to Soka University, she began research on glycobiology. She is currently Vice President of Japan Consortium for Glycobiology (JCGG) and an Editorial Board member of the Glycoconjugate Journal.
Research theme: She is focusing on the function of glycans that are conserved across species. She uses comprehensive screening focusing on the functions to find and analyze the functions of glycans in mammalian pluripotent stem cells and in the Drosophila model. In addition, recently, she has started joint research to develop screening methods for identifying undiagnosed and rare diseases related to glycans.
Cell surface glycans are tissue-specific and developmentally regulated; therefore, they are used as markers of embryonic stem cells (ESCs) such as stage-specific embryonic antigens. However, the role of glycans in stem cells has not been fully elucidated. Therefore we performed an RNA interference (RNAi) screen for glycosyltransferases essential for self-renewal and pluripotency of mouse ESCs and identified the following four glycan structures that are required to maintain the naïve pluripotent state: (1) LacdiNAc structure (GalNAc β1,4GlcNAc), (2) heparan sulfate (HS), (3) O-GlcNAc, (4) T antigen (Galβ1,3GalNAc). They regulate the leukemia inhibitory factor (LIF), bone morphogenetic protein (BMP), and/or Wnt signals that act to maintain the naïve pluripotent state and/or the fibroblast growth factor 4 (FGF4) signal that triggers differentiation. Thus, various types of glycans regulate the stem cell status; these glycan structures are evolutionarily conserved from Drosophila to mammals. In addition, ESCs and epiblast-like cells recapitulate in vitro the epiblast first cell lineage decision, allowing characterization of the molecular mechanisms underlying pluripotent state transition. Comprehensive and comparative analysis of the total glycomes of both cells revealed that overall glycosylation undergoes dramatic changes from the early stages of development. We also showed the presence of a developmentally regulated network orchestrating glycosylation changes and identified polycomb repressive complex 2 as a key component involved in this process. Here, I would like to introduce our work on stem cells, in particular, glycan functions in stem cells and the integrated control of glycan structure expression in stem cells, including attempts to apply glycans to regenerative medicine.
Mammalian pluripotent stem cells such as ESCs and induced pluripotent stem cells (iPSCs) are in two different states, the so-called naïve and primed states1-3, and a more recently formative state between naïve and primed states has been proposed4. The naïve state corresponds to the pre-implantation blastocyst stage of the embryo and the primed state corresponds to the post-implantation blastocyst stage. Both states show different characteristics. Mouse ESCs derived from the inner cell mass of 3.5-day-old embryos are in the naïve state, whereas conventional human ESCs are in the primed state based on their charactistics1-3.
Many of the markers for mammalian pluripotent stem cells such as ES and iPS cells are glycans2,3,5. Mouse ESCs express stage-specific embryonic antigen-1 (SSEA-1) on both glycoproteins and glycolipids, whereas conventional human ESCs express SSEA-3,4,5 but not SSEA-1. However, the biological functions of glycans in pluripotent stem cells were not well understood. Therefore, we performed an RNAi screen for more than 100 glycosyltransferases in naïve mouse ESCs, using alkaline phosphatase activity to identify and evaluate glycans that are essential for ESC self-renewal. To date, we have found four different types of glycan structures required for maintenance of the naïve pluripotent state; they regulate signals upstream of pluripotency-associated transcription factor network or inhibit the signal that triggers differentiation (Fig. 1).
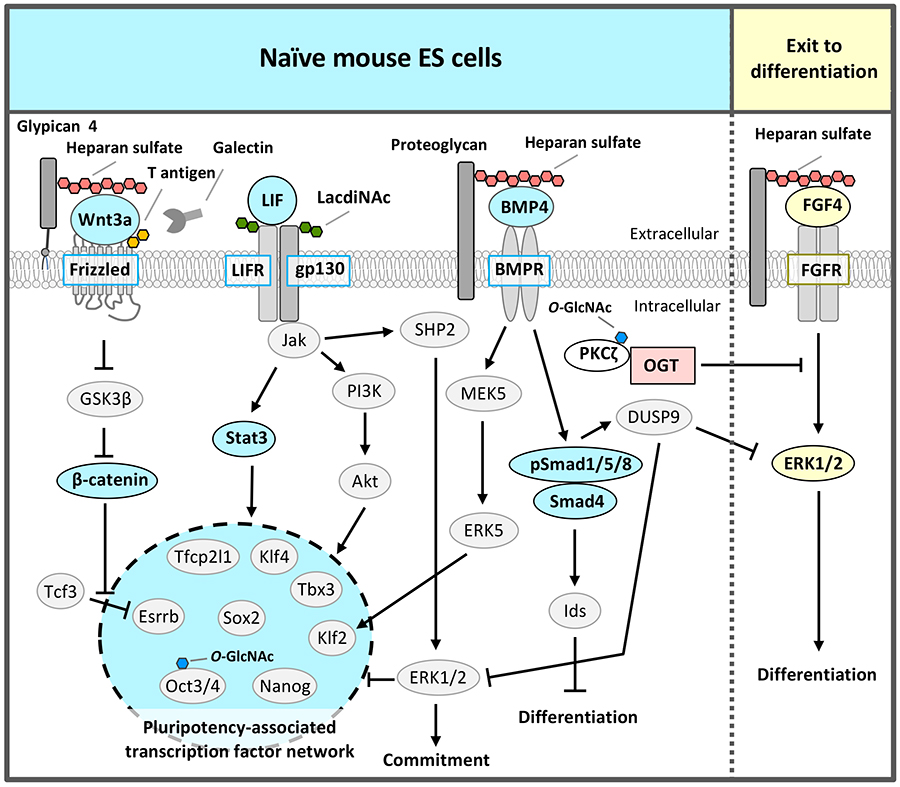
In the naïve state, the pluripotency-associated transcription factor network is composed of Nanog, Sox2, Oct3/4, Klf4, Klf2, Tbx3, Tfcp2l1, and estrogen-related receptor b (Esrrb) that regulate each other and collaborate to regulate downstream gene expression to maintain self-renewal and pluripotency (Fig. 1). As the LIF signal induces the expression of these transcription factors through activation of the Jak/Stat3 and PI3K/Akt pathways6, LIF needs to be added to the culture medium of naïve pluripotent stem cells. The Jak/Stat3 pathway directly activates Klf4 and Tfcp2l1, and the PI3K/Akt pathway directly activates Tbx3.
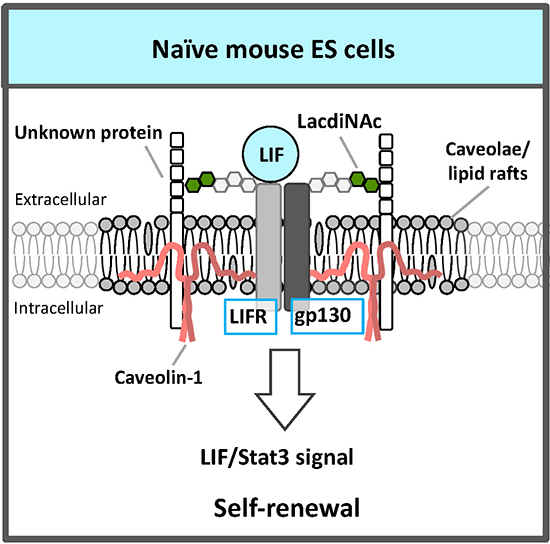
An RNAi screen for glycosyltransferases essential for self-renewal and pluripotency in mouse ESCs identified β1,4 N-acetylgalactosaminyltransferase 3 that synthesizes the LacdiNAc (GalNAcβ1,4GlcNAc) structure7. LacdiNAc, which is a glycan structure conserved from Drosophila to humans, is a unique terminal structure in the outer chain moieties of N-glycans or O-glycans. LacdiNAc on the LIF receptor (LIFR) and glycoprotein 130 (gp130) is required for maintenance of the naïve state via LIF/Stat3 signaling (Fig. 2). LacdiNAc expression is high in naïve mouse ESCs and decreases during differentiation. In the naïve state, LacdiNAc on LIFR and gp130 contributes to interaction with the caveolin-1 complex components of caveolae/lipid rafts; this interaction stabilizes LIFR and gp130 localization to the rafts (Fig. 2). A lipid raft is a microdomain within the lipid bilayer of the cellular membrane that is enriched in glycosphingolipids, sphingomyelin, and cholesterol, and has transmembrane or glycosylphosphatidylinositol-anchored proteins. Lipid rafts are indispensable for cellular signaling processes because many signal ligands, receptors, and Src family kinases are assembled there. Therefore, localization of LIFR and gp130 to caveolae/lipid rafts promotes the complex formation of LIFR and gp130, which is necessary for LIF signal transduction, and the signal is transmitted efficiently.
In addition to LIF signal, the BMP4/Smad and mothers against decapentaplegic homolog (Smad) and Wnt/β-catenin signals are also required for self-renewal and pluripotency in naïve pluripotent stem cells (Fig. 1)1-3. Wnt signaling synergistically with LIF promotes the maintenance of self-renewal via Esrrb activation, although Wnt signaling alone is insufficient to maintain self-renewal of naïve mouse ESCs. Drosophila mutant analysis8 and the strong binding of heparin, which has similar structure of HS, to BMP4 (Kd = 69.4 nM)9 and Wnt3a (Kd = 26.0 nM)10 indicate that HS functions as a co-receptor or stabilizing factor of BMP and Wnt.
The RNAi screen of glycosyltransferases required for self-renewal and pluripotency of naïve mouse ESCs also identified exostosin 1 (EXT1), which synthesizes the disaccharide repeat region of HS10. Analyses by several groups, including ours, showed that HS regulates Wnt and BMP signaling to maintain the naïve state of ESCs (Fig. 1)2,3. In this case sulfation of HS was indispensable9. Furthermore, among HS proteoglycans, glypican 4 selectively regulated Wnt signaling11. HS covalently binds to various core proteins to form HS proteoglycans. The contributions of HS to Wnt signaling might depend on core proteins.
O-GlcNAc is the only type of glycosylation that occurs in the cytoplasm, nucleus, or mitochondria. O-GlcNAc transferase (OGT) transfers a single GlcNAc to serine (Ser) /threonine (Thr) residues of proteins, whereas O-GlcNAcase (OGA) removes O-GlcNAc from proteins12. Proteins modified by O-GlcNAc include signaling components, transcription factors, epigenetic regulators, and histones. Thus, O-GlcNAc has various functions such as inhibition of phosphorylation, regulation of transcriptional activity, stabilization of proteins, and regulation of intracellular localization.
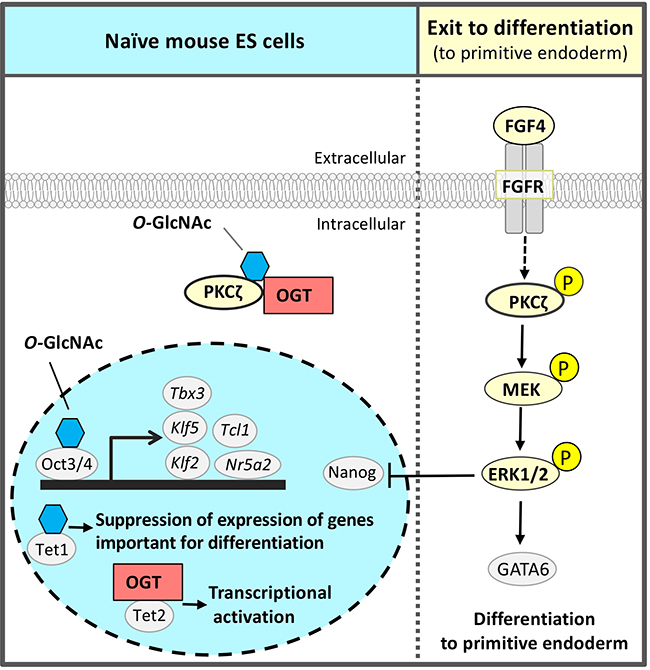
The third glycan identified by the RNAi screen of glycosyltransferases required for self-renewal and pluripotency of naïve mouse ESCs was O-GlcNAc 2,13,14 (Fig. 3). O-GlcNAc modification is often performed at or near the Ser / Thr residue to be phosphorylated; it is considered that O-GlcNAc modification and phosphorylation are competitive with each other. The FGF4 signal is an important signal triggering differentiation. It suppresses the pluripotency-associated transcription factor network of naïve mouse ESCs and promotes differentiation (Fig. 1 and 3). We hypothesized a mechanism that suppresses FGF4 signal to maintain the naïve undifferentiated state of mouse ESCs and focused on inhibition of phosphorylation by O-GlcNAc. FGF4 signal activates the phosphorylation of the mitogen-activated protein kinase kinase (MEK) - extracellular signal-regulated kinase 1/2 (ERK1/2) pathway downstream of the FGF receptor. Phosphorylated ERK1/2 suppresses the expression of Nanog, which is a transcription factor essential for maintaining the naïve undifferentiated state (Fig. 3). In Ogt knocked down (KD) cells, the FGF4-MEK-ERK1/2 pathway was activated, reducing the expression of markers of the undifferentiated state and promoting differentiation. On the other hand, in naïve mouse ESCs, Thr410 of protein kinase c ζ (PKCζ), which is a FGF4 signal component, was found to be modified by O-GlcNAc. PKCζ is activated when Thr410 is phosphorylated and then activates the downstream MEK-ERK1/2 pathway. In naïve mouse ESCs, Thr410 is modified by O-GlcNAc to inhibit its phosphorylation and the activation of PKCζ, resulting in suppression of FGF4 signal14 (Fig. 3). That is, O-GlcNAc suppresses the FGF4 signal and contributes to the maintenance of the naïve undifferentiated state.
In addition to regulation of signal components, O-GlcNAc has also been reported by other groups to regulate transcription and epigenetic factors in pluripotent stem cells2. Transcription factors involved in the pluripotency-associated transcription factor network, Oct4 and Sox2, are modified by O-GlcNAc in naïve mouse ESCs, which decrease upon differentiation15. O-GlcNAc modification of Thr228 in Oct4 positively regulated Oct4 transcriptional activity and was important for the induction of genes essential for maintaining naïve status, such as Klf2, Klf5, Nr5a2, Tbx3, and Tcl1 (Fig. 3). Ten-eleven-translocation (Tet) 1 was stabilized by O-GlcNAc modification, formed a complex with Sin3A and NuRD leading to an inhibitory chromatin structure, and suppressed the expression of genes important for differentiation16. On the other hand, Tet2 recruited OGT to histone H2B, and O-GlcNAc modification of Ser112 in histone H2B activated transcription17. Thus, O-GlcNAc had a wide variety of functions and worked to maintain naïve mouse ESCs.
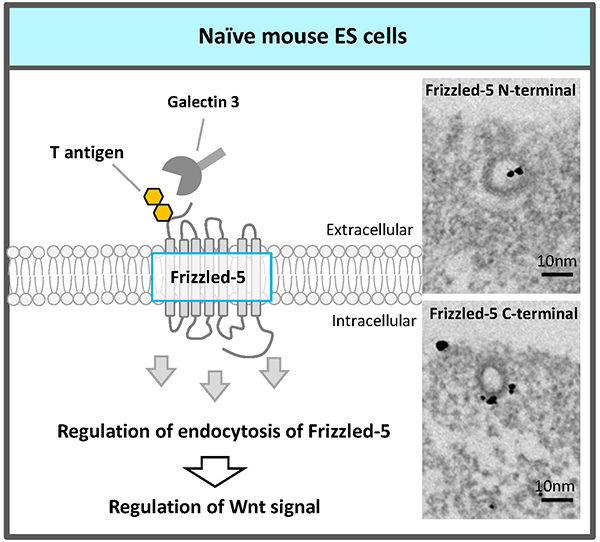
Recently, an RNAi screen of glycosyltransferases required for self-renewal and pluripotency of naïve mouse ESCs identified the fourth glycosyltransferase, core 1 β1,3-galactosyltransferase (C1GalT1) which synthesizes T antigen (Galβ1,3GalNAc), one of most abundant mucin type O-glycans18. The elongation pathway via C1GalT1 was the most prominent among mucin-type O-glycosylation modifications in naïve mouse ESCs. C1GalT1 KD resulted in the loss of ESCs pluripotency and differentiation to trophectoderm. Moreover, we found that mucin-type O-glycosylation on Frizzled-5 directly regulates its endocytosis via galectin-3 binding to T antigen on Frizzled-5, and that reduction of T antigen results in the exit of the ESCs from pluripotency via canonical Wnt signaling excess activation (Fig. 4). It reveals a novel regulatory mechanism that modulates Wnt signaling and, consequently, ESC pluripotency.
As mentioned above, four types of glycans regulate the stem cell status. These glycan structures are evolutionarily conserved from Drosophila to mammals, probably because the prototype of the regulatory mechanism of cell signaling by glycans is conserved across species.
Glycosyltransferases, of which there are more than 200 in humans, are responsible for synthesizing the huge diversity of complex oligosaccharides; glycan structures on proteins and lipids in each species can be estimated by analysis of their glycosyltransferases. During evolution from yeast to mammals, construction of fundamental gene families of glycosyltransferases was complete before divergence of deuterostomes and protostomes including C. elegans and Drosophila19. Therefore, it is considered that essential glycan structures are conserved across species.
Drosophila provides well-established models of mammalian tissue stem cells, such as germline, intestinal, hematopoietic, and neural stem cells2,19. Tissue stem cells require a particular environment, which is called the stem cell niche. To maintain tissue stem cells, extrinsic signals from the stem cell niche, as well as intrinsic regulatory factors, are required. Tissue stem cells divide asymmetrically. The daughter cell that is a niche resident receives extrinsic signals from the niche and becomes a stem cell, while the daughter cell that is not a niche resident begins to differentiate into a mature tissue cell. Many groups reported that HS proteoglycans contribute to BMP signals from the stem cell niche to germline stem cells20 and to signals, such as epidermal growth factor (EGF), hedgehog (Hh), and FGF for intestinal, hematopoietic, and neural stem cell proliferation and maintenance19.
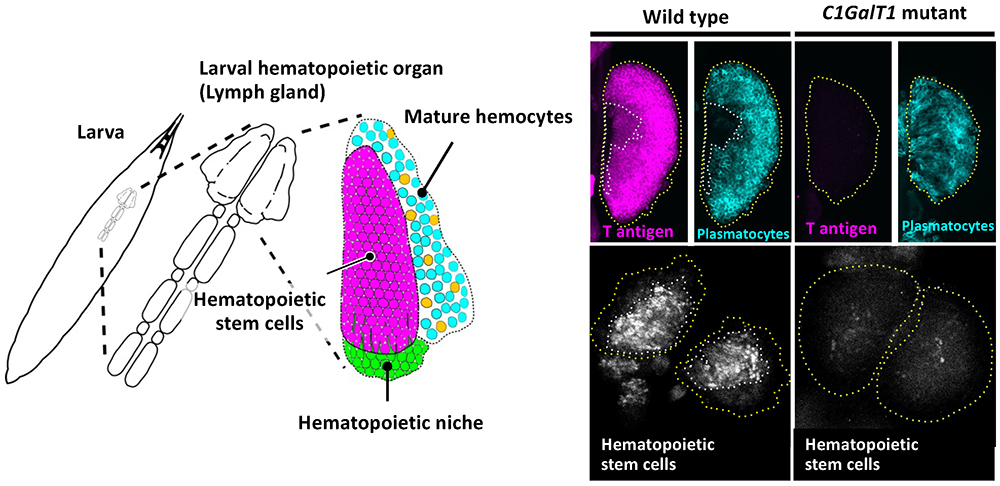
During analysis of glycan functions conserved between Drosophila and humans, we found that T antigen (Galβ1,3GalNAc), one of the mucin type O-glycans, is required for maintenance of hematopoietic stem cells (HSCs)21. Hematopoiesis in Drosophila occurs in two stages, embryonic and larval. Larval hematopoiesis occurs in the lymph gland just beneath the dorsal epidermis. The primary lobe of the lymph gland contains three regions: the hematopoietic niche, hematopoietic stem cells, and mature hemocytes (Fig. 5, left panel). In Drosophila, mature hemocytes, corresponding to vertebrate myeloid cell lineages, consist of three types of cells: plasmatocytes, crystal cells, and lamellocytes. Plasmatocytes, corresponding to vertebrate macrophages, comprise more than 95% of total mature hemocytes and function in cellular immunity and phagocytosis.
Reduced T antigen causes loss of HSCs in the lymph gland in Drosophila C1GalT1 (dC1GalT1) mutant larvae21 (Fig. 5, right panel). Hemolymph has an important role in creating a favorable environment for the hematopoietic organ, because every larval organ is immersed in hemolymph. The mucin type O-glycan T antigen on hemolectin (Hml), which is secreted from plasmatocytes to hemolymph and involved in hemolymph coagulation, determines the biochemical characteristics of hemolymph and creates conditions that are favorable to filopodial extension. Reduced T antigen alters the characteristics of hemolymph or Hml itself, allowing the clotting factor Hml to form a shell around the lymph gland, resulting in the loss of hematopoietic stem cells through impairment of filopodial extension from the hematopoietic niche.
As mentioned above, both the HS proteoglycan and mucin type O-glycan T antigen also have important roles in maintenance of Drosophila tissue stem cells as well as in maintenance of mouse naïve pluripotent stem cells.
Mouse ESCs are derived from pre-implantation embryos at E3.5-E4.522, whereas mouse epiblast-like cells (EpiLCs), which differentiate from ESCs in culture, resemble post-implantation embryos at E5.5-E6.523. ESCs and EpiLCs reflect two distinct pluripotent states known as the naïve state and the primed state, respectively, providing a useful model system to examine the pluripotent state transition occurring at implantation in vitro24.
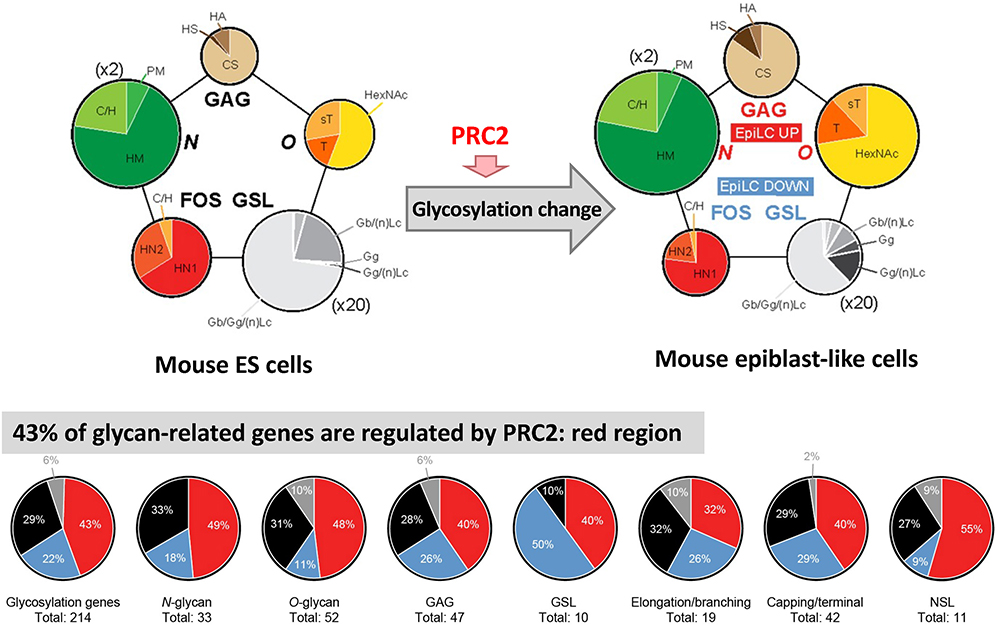
For mouse ESCs and EpiLCs, we performed total glycome analyses to determine the absolute amounts of all types of glycan structures such as N-glycans, O-glycans, glycosaminoglycans (GAGs), glycosphingolipids (GSLs), and free oligosaccharides (FOSs)25. Using the same samples used for total glycome analysis, we also performed quantitative gene expression analysis of all glycosyltransferases in mouse ESCs and EpiLCs. All glycosylation types undergo dramatic changes, both at the transcriptional and the structural level, from early stages of development (Fig. 6). The identification of novel specific structures in each cell across all glycosylation types provided additional markers to distinguish between the naïve and primed pluripotent states. Compared to other types of cells such as human ESCs and normal/carcinoma somatic cell lines26, both mouse ESCs and EpiLCs showed a typical comprehensive glycomic profile; there was greater GSLs expression compared to that of other types of glycans, although GSLs expression decreased during the transition from ESCs to EpiLCs (Fig. 6). FOSs also decreased during transition, whereas N-glycans, O-glycans and GAGs increase.
PRC2 deposits three methyl groups at histone H3 lysine 27 (H3K27me3) to promote gene repression27. We identified PRC2 as one key component involved in the network orchestrating the dramatic glycosylation changes during ESC to EpiLC transition.
At first, we performed an in-depth analysis of previously published chromatin immunoprecipitation sequencing (ChIP-seq) datasets obtained in ESCs using the ChIP-Atlas comprehensive database (https://chip-atlas.org)28, searching for factors that are enriched at the promoter regions of glycosylation-related genes including glycosyltransferases. Then we found that PRC2 components occupy various glycosylation-related genes across all glycosylation types in ESCs and confirmed it by experiments using ESCs treated with the PRC2 inhibitor EED226 that induced considerable reduction of the H3K27me3 modification25.
Comparing the glycosylation alterations observed by fluorescence-activated cell sorting during the ESC to EpiLC transition and in ESCs after EED226 treatment, many structures were increased or decreased both in EpiLCs and EED226-treated ESCs, indicating a direct modulation by PRC2. To further confirm it, we also compared the difference in glycosyltransferase and related enzyme expression between ESCs vs EpiLCs and EED226 untreated vs EED226 treated samples by RNA-seq, and demonstrated that expression in 43% of the genes was increased or decreased both in EpiLCs and EED226-treated ESCs (Fig. 6). These results demonstrate “the presence of a developmentally regulated network orchestrating overall glycosylation changes by PRC2”.
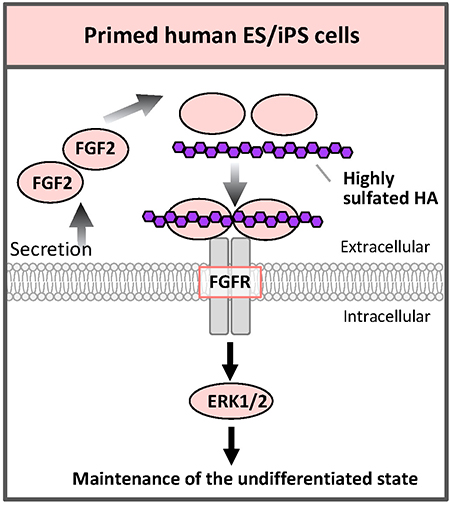
Comparing the absolute amounts of the types of glycans expressed in human ESCs/iPSCs, the amounts of sulfated GAGs such as HS or chondroitin sulfate (CS) are more than half of the total glycans amount26. The function of GAGs on primed human ESCs/iPSCs is not well understood, but, in fact, FGF2 and heparin are added in serum-free medium29. HS secreted by feeder cells or synthesized by ESCs/iPSCs themselves binds to FGF2 and stabilizes FGF2; it is important for proliferation and maintenance of ESCs/iPSCs.
Hyaluronic acid (HA) is a kind of GAG and is composed of repeating disaccharide units of glucuronic acid and N-acetylglucosamine. However, but unlike other GAGs, HA is not sulfated. We have recently succeeded in artificially sulfating HA and freely adjusting the degree of sulfation. Highly sulfated HA (3.63 for the maximum 4 hydroxyl groups per disaccharide unit) binds FGF2 more strongly than heparin. When human iPSCs were cultured with highly sulfated HA that was added to the medium, human iPSCs could be cultured with pluripotency even under feeder-cell-free conditions, no addition of FGF2, and no serum30 (Fig. 7). In fact, the addition of highly sulfated HA also enhanced the FGF signal. It is considered that highly sulfated HA binds to more than 2 molecules of FGF2 to promote dimer formation of the FGF receptor, resulting in enhanced signaling. We also synthesized low sulfated HA. By properly using these HAs with different degrees of sulfation, the functions of GAGs such as HS and CS in vivo can be mimicked without contamination by biomolecules from other species; in various aspects of regenerative medicine, it will be helpful to develop conditions of differentiation that are closer to the ones found in vivo during the developmental process. It would be possible to regulate various signals safely.
As mentioned above, various types of glycans on the stem cell surface regulate the reception of major external signal ligands and are involved in the maintenance of stem cell pluripotency and differentiation. Furthermore, O-GlcNAc, the only intracellular glycan modification, regulates the phosphorylation of downstream signaling molecules, which is also involved in the maintenance of stem cell pluripotency and differentiation. The functions of glycans described here are some of the glycan functions that we clarified in our previous work; glycans have many more functions, including functions that have not yet been elucidated. Understanding these glycan functions and applying this understanding to solving problems in various life sciences including stem cell biology and embryology will contribute to the development of treatments in many medical fields such as intractable diseases, rare diseases, and cancer, as well as regenerative medicine.