
Ken-ichi Kurotani
Ph.D.
Project Lecturer, Bioscience and Biotechnology Center, Nagoya University.
He earned his Ph.D. in the Graduate School of Agriculture, Kyoto University in 2002. Having been a postdoctoral fellow at Nara Institute of Science and Technology (NAIST), Kyoto University, and Nagoya University, he has been in his current position from 2019. His research interests include environmental responses and signal transduction in plants. He continues to approach plant science from both a dry and wet perspective with a focus on bioinformatics.

Michitaka Notaguchi
Ph.D.
Associate Professor, Bioscience and Biotechnology Center, Nagoya University.
He earned his Ph.D. in the Graduate School of Science, Kyoto University in 2009. He studied at the University of California, Davis, and was a JSPS Postdoctoral Fellow. 2012: Research Fellow, Graduate School of Science, Nagoya University, and JST ERATO Higashiyama Live Holonics Project Research Fellow. 2015: Project Assistant Professor, Graduate School of Science, Nagoya University, and JST PRESTO Research Fellow. 2016: Assistant Professor, Graduate School of Bioagricultural Sciences, Nagoya University; Collaborative Researcher, Institute of Transformative Bio-Molecules (ITbM), Nagoya University; Leading Initiative for Excellent Young Researchers, MEXT. Since 2019, he has been in his current position. He is working on the mechanism of plant grafting and systemic signal transduction.
Grafting is a technique for cultivating plants that combines the advantages of two different plant species, and it has been used as an agricultural technique since ancient times. The cell wall surrounding the plant cell is an extracellular matrix composed of multiple polysaccharides, the composition of which depends on the plant species. Grafting is thought to occur when the cell walls of two grafted plants are reconstituted at their boundaries, resulting in cell or tissue adhesion. In this article, we will introduce the mechanism of artificial plant grafting and the similarity between grafting in nature and grafting in plants, focusing on the function of digestive enzymes of cellulose, the main component of cell walls.
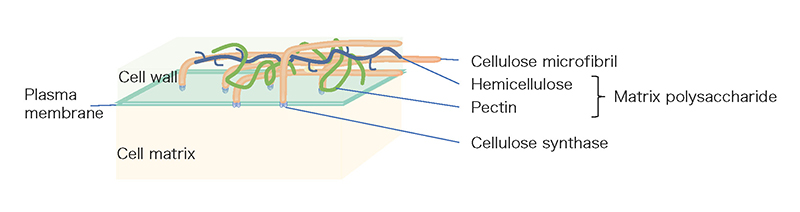
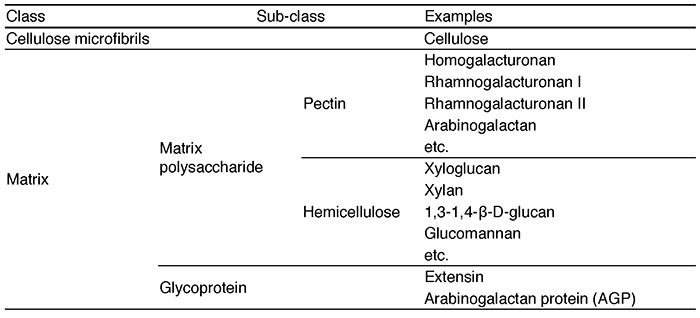
The grafting technique combines a subterranean part (rootstock) of one plant that is well adapted to the soil characteristics with an above-ground part (scion) of a second plant that is more productive and is currently used to improve the characteristics of various crops. Grafting has a history of more than two thousand years and most of existing fruit trees such as apple, pear, peach, vine and mandarin are produced by grafting 1. This technology is essential for fruit productivity, resistance to soil diseases, and good crop quality. In recent years, grafting has also been used for vegetables such as tomatoes, cucumbers, and eggplants, and the majority of these vegetables on Japanese tables are harvested from plants grown on grafted seedlings. However, there are some major limitations to grafting that impede the agricultural use of the grafting technique. In general, grafting can only be established between plants with closely related genetic backgrounds. For example, when plants belonging to different families, such as rose and chrysanthemum, are grafted together, the tissue at the junction does not adhere and the scion dies or drops to the ground 2. The process of grafting is accomplished by cell proliferation on the surface of the injured stem, adhesion of the cells and tissues of the two plants to each other, and tissue reorganization. This initial cell-tissue adhesion is thought to require reconstitution of the cell walls of the two plants. One of the major characteristics of plant cells, in contrast to animal cells, is the presence of cell walls (Fig. 1). The cell wall of land plants plays a major role in the formation of plant morphology 3. It consists of a cellulose backbone and a substrate containing polysaccharides (classified as pectins and hemicelluloses) and proteins. Cellulose is a linear sugar chain consisting of thousands to tens of thousands of polymerized β-D-glucose molecules. Several dozens of these cellulose molecules are arranged in parallel and bundled together to form the basic fiber, and another ten or so cellulose microfibers form the framework of the cell wall. On the other hand, the polysaccharides that make up the matrix are called matrix polysaccharides, and they consist of a wide variety of polysaccharide components such as homogalacturonan, rhamnogalacturonan I, rhamnogalacturonan II, xyloglucan, β-glucan, xylan, and glucomannan and so on4 (Table 1). In distantly related plants, the components of the cell wall are different, making it difficult to reconstitute, and the initial event of grafting, cell or tissue adhesion, may not be sufficient. In fact, in most cases of interfamily grafting, tissue adhesion does not occur after grafting, and necrosis of the grafted stem surface has been observed.
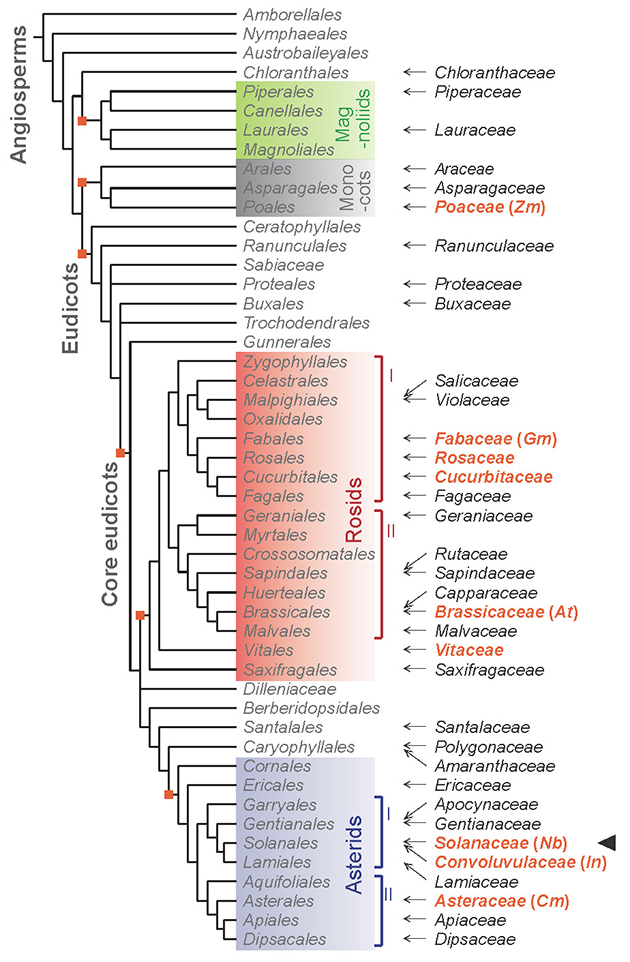
Our group has been studying the mechanism of distant signal transduction in plants for a long time, and we used grafting as an experimental technique to verify the transport and movement of signal substances using model plants such as Arabidopsis. In this study, we found that when Nicotiana benthamiana, a model plant of the Nicotiana genus in the Solanaceae family, and Arabidopsis, a model plant for molecular genetics research, were grafted, the connection sites were firmly attached and the scion survived for a long time despite the genetic distance of their families5. Extending the combination of interfamily grafting to a variety of plant species in the angiosperms, we performed grafting on seven plant species of the Nicotiana genus, including N. benthamiana, with 84 plant species in 42 families. We found that they could be grafted with 73 plant species in 38 families (Figs. 2 and 3). In the long history of grafting, such a high degree of grafting capability has never been found before, and the amazing ability to graft onto plants in different families was found in the plants of the Nicotiana genus.
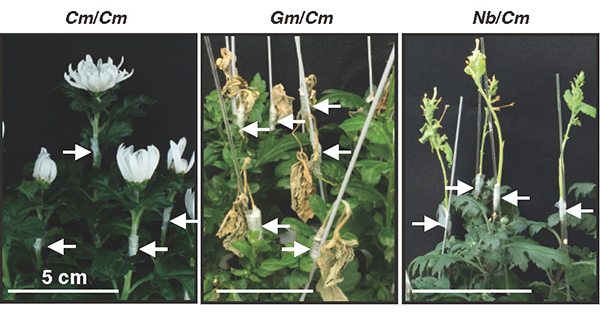
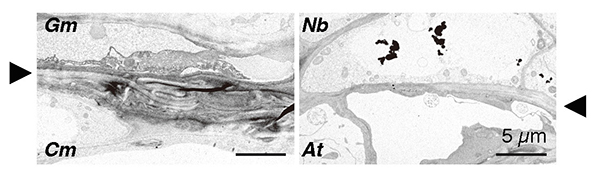
So, what is the difference between tobacco plants that can be grafted with different families and other plants that cannot? To get a clue, we first examined morphologically the graft junction. Microscopic observation of tissue sections revealed that the parenchyma tissues, called the callus, proliferated from the grafted parts of N. benthamiana through cell division. These parenchyma tissues adhered well to the plant tissues of the graft partner, and only a thin layer of necrosis was observed, which is frequently observed when grafting fails in other interfamily grafts. More detailed observation of the cells using a transmission electron microscope showed that in the case of Glycine max (Gm, soybean) and Chrysanthemum morifolium (Cm), grafting was not successful and broken cell remnants such as cell walls were trapped at the graft interface, forming a necrotic layer; in the case of N. benthamiana (Nb) and Arabidopsis (At), grafting was successful and the cells were in close proximity to each other and the cell walls of the living cells of both plants were in close contact (Fig. 4). This suggests that the decomposition of cell debris and cell adhesion between the two plants may be important factors in the establishment of grafting.
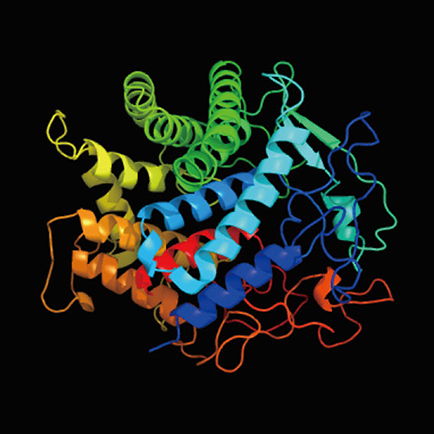
Next, we performed time-series transcriptome analysis of the grafted samples of N. benthamiana and Arabidopsis by RNA-seq analysis using a next-generation sequencer to explore the molecular mechanism of interfamily grafting. Since the results of microscopy observation suggested that the cells of the two plants started to fuse together on the third day after grafting, we extracted genes whose expression remained elevated on the first, third, and seventh days after grafting, and 189 genes were identified. Gene ontology analysis of these genes showed enrichment of genes related to the extracellular components, cell wall, apoplast, and plasmodesmata. On the other hand, the same time-series transcriptome analysis was performed, for comparison, on plant samples of soybean and Arabidopsis grafted parts, where interfamily grafting was not established. Genes in soybean homologous to 189 genes identified in N. benthamiana were extracted by genome analysis and their gene expression patterns were evaluated when soybean was grafted with plants of different families. In order to extract genes that are specific to N. benthamiana and enable the establishment of interfamily grafting, we narrowed down the candidate genes to those whose expression does not increase in soybean. In fact, 79 genes were narrowed down from 189 genes. Among these candidate genes, a member of the GH9B3 subfamily of β-1,4-glucanase in plants was targeted for further analysis. β-1,4-glucanase, also called cellulase, is a secreted enzyme protein that is suggested to be involved in cell wall modification (Fig. 5). Cellulase is an endo-type degrading enzyme and is thought to cleave the sugar chains of cellulose, xyloglucan, β-glucan, and glucomannan. When Arabidopsis plants were grafted with N. benthamiana plants in which cellulase gene expression was transiently suppressed, the grafting success rate was reduced compared to that of untreated plants. These results suggest that the function of cellulase genes of the GH9B3 subfamily is important for interfamily grafting success in N. benthamiana.
Grafting, whether in a different family or the same family, is an artificial manipulation and is not considered a naturally occurring process. So, what is the original function of the GH9B3 cellulase gene? We hypothesized that the cellulase may play a role in the repair of accidentally occurring stem damage and breakage. To investigate this possibility, we first generated N. benthamiana plants in which the GH9B3 cellulase gene was disrupted using CRISPR-Cas9 and grafted the plants onto themselves. As a result, we found that the success rate of grafting was significantly lower in grafted plants with disrupted GH9B3 cellulase genes than in grafted wild-type plants. Next, soybean, morning glory, maize, and Arabidopsis were also grafted onto themselves, and the expression patterns of the β-1,4-glucanase gene cluster were analyzed over time. The expression of cellulase genes was also found to be up-regulated in soybean, morning glory, and Arabidopsis during self-grafting. This up-regulation was characteristic of cellulase genes belonging to the GH9B3 subfamily. In addition, in Arabidopsis, grafting of the cel3, a mutant of the GH9B3 cellulase gene, resulted in poor growth after grafting; conversely, artificially overexpressing the GH9B3 cellulase gene at the grafting site increased the success rate of grafting. These results support the possibility that GH9B3 cellulase functions universally in the grafting of a wide range of plants in the same family. On the other hand, when soybean, morning glory, and maize were grafted onto Arabidopsis, the expression of GH9B3 cellulase was transiently up-regulated in soybean and morning glory, but the expression did not remain beyond a certain level. We found that 1) the expression of the GH9B3 cellulase gene is up-regulated when plants are grafted, and the upregulated expression promotes tissue fusion at the jointed surface; 2) the expression of the GH9B3 cellulase gene is not maintained in common plants when the grafting partner does not belong to the same family, and thus fusion is not possible; and 3) in the case of tobacco plants, the expression of GH9B3 cellulase gene is maintained even when the grafting partner is not a member of the same family. The lack of expression in maize, a monocotyledonous plant, is thought to be due to the loss of the repair mechanism, since monocotyledonous plants do not repair damage to their stems.
Cellulases do not directly catalyze cell wall fusion, but by cleaving cellulose, which is a major component of plant cell walls, they may provide material for cellulose microfiber reconstruction and fusion between the rootstock and scion. Another hypothesis is that degraded cell wall-derived glycoconjugates trigger the expression of some genes to promote tissue fusion and this hypothesis will be clarified in future studies.
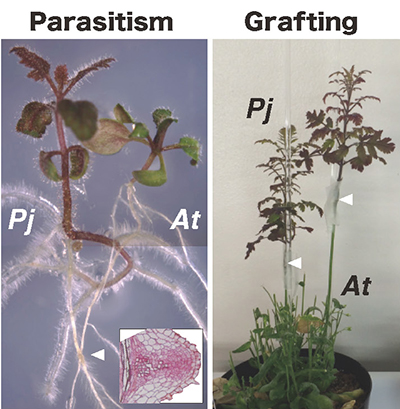
Here is another related finding from a study that focused on parasitism. Whereas grafting is an artificial joining of plants, parasitism is a natural mode of association with heterologous plant tissues acquired by the parasitic plant. We hypothesized that parasitism and interfamily grafting of Nicotiana plants might share a common mechanism of heterologous bonding, and we decided to test this hypothesis. We first observed the morphology at the site of parasite attachment in Phtheirospermum japonicum, which is used as a model in the study of parasitic plants. It forms a haustorium, a sucker organ, on its roots and parasitizes the roots of host plants. Electron microscopy of the host-parasite interface showed that the cell wall in contact with the host plant tissue was locally reduced in thickness by digestion, similar to the interface at the interfamily graft junction with N. benthamiana. If there is a common mechanism underlying parasitism and interfamily grafting, we thought that parasitic plants should also establish interfamily grafting, and we carried out grafting of P. japonicum to Arabidopsis. As a result, it was found that parasitic plants, as happened with N. benthamiana, can also form interfamily grafting 6 (Fig. 6). These results suggested that parasitism and interfamily grafting share a common molecular mechanism, so we conducted a comparative analysis of the transcriptomes of the tissues in the parasitic haustorium and junction of P. japonicum and Arabidopsis grafting junctions. Gene clusters with up-regulated expression at the time of parasitism and those with up-regulated expression at the time of tissue fusion by interfamily graft formation were extracted by clustering using a self-organizing map. We found that the GH9B3 cellulase gene was included in the common cluster. Furthermore, when the expression of the GH9B3 cellulase gene was transiently suppressed in the haustorial apparatus, the rate of abnormalities in the formation of parasite-derived structures increased, strongly suggesting that β-1,4-glucanases belonging to the GH9B3 subfamily also play a role in parasitism.
Although grafting is an artificial process, it may be equivalent to the repair of naturally-occurring accidental injuries. When repairing damaged tissues, there may be a self-recognition mechanism that allows the plant to heal itself if the tissues to be united are its own, but to reject the opposing tissue partner if the tissues to be united are not its own. For some reason, Nicotiana plants may have evolved in a direction that loosens constraints imposed by this self-recognition, while parasitic plants may have evolved in a way that allows them to associate with other plants.
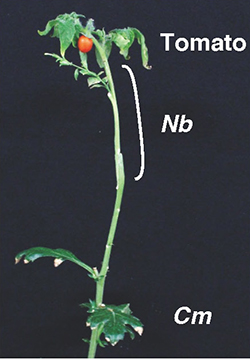
Although the grafting technique combines the characteristics of two plants, the Nicotiana genus has the potential to extend the application of grafting to the plants of different families. Based on this discovery, we hypothesized that by using Nicotiana plants as an intermediate rootstock, it would be possible to indirectly graft different families of distantly related plants that are not normally able to form a graft union. We decided to use the most prosperous terrestrial plant, Asteraceae, as the rootstock and tomatoes, the most commonly eaten vegetable in the world, as the scion. By placing an N. benthamiana plant between these two plants, the grafting of Chrysanthemum, N. benthamiana, and tomato was successfully completed and the seedlings grew for more than three months and produced tomato fruits (Fig. 7). Asteraceae plants are highly fertile and resistant to various soil stresses. In the future, we hope to be able to utilize such a robust plant resource through a method using interfamily grafting.
The interfamily grafting method is expected to be used for not only crop production but also delivery of biomolecules such as plant hormones, proteins, and RNAs through the graft. So far, we have succeeded in inducing gene silencing and protein expression in the grafted plant via interfamily grafting, and we believe that interfamily grafting may be used as a means of indirectly exploiting genetic modification technology.
At present, tissue union is still incomplete and not sufficient for scion growth in interfamily grafting compared to homologous grafting. We hope that the discovery of the GH9B3 cellulase gene will lead to the development of technology that can be used to create stronger tissue bonds. In fact, when the effects of the external application of cellulase were assessed, it was experimentally shown that the physical adhesion of tissues at the graft site was improved 7. By focusing on the ability of Nicotiana plants to graft with plant tissues belonging to different families, we hope to gain a better understanding of the processes involved in grafting, such as cell proliferation, self-recognition, and re-organization of the vascular tissues. By addressing these important issues in plant science, we hope to improve grafting technology and the utilization of plant resources, and help address the global environmental problems caused by recent global population growth and associated human activities.
Acknowledgments
The research results presented in this paper were obtained through the efforts of many researchers and related groups, and the research itself has been supported by JST (START 15657559, PRESTO 15665754), Canon Foundation (R17-0070), Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (18KT0040, 18H03950, 19H05361), National Institute of Agrobiological Sciences (NIAS) under the Research Promotion Program for Enhancing Innovation (28001A, 28001AB). We would like to express our sincere gratitude to all of them, and we hope to receive their guidance and support for further promotion of grafting research and dissemination of the technology.