
Koji Ogawa
Assistant professor, Department of Gastroenterology and Hepatology, Hokkaido University. MD and PhD.
I graduated from Faculty of Medicine, Osaka City University in 1997. After clinical training of gastroenterology, mainly liver disease at several hospitals in Hokkaido, I have been conducting clinical research at Hokkaido University Hospital since 2014. Currently also serves as director of the Hokkaido University Hospital Liver Disease Consultation Center.
Research Interest : I am doing clinically research related to liver disease under the guidance of Professor Naoya Sakamoto. In addition, I am also conducting research on glycan analysis in NAFLD and other liver diseases.

Naoya Sakamoto
Professor, Department of Gastroenterology and Hepatology, Graduate School of Medicine, Hokkaido University. MD, PhD.
I graduated from Faculty of Medicine, Tokyo Medical and Dental University in 1987. After clinical training focusing on gastroenterology and hepatology, I studied abroad as a research fellow at Connecticut State University School of Medicine from 1994. After returning from studying abroad, I worked at Gastroenterology and Hepatology, Tokyo Medical and Dental University, and I have been a professor at Gastroenterology and Hepatology, Graduate School of Medicine, Hokkaido University since 2012.
Research Interest : I conduct research on liver diseases such as viral hepatitis, NAFLD, liver fibrosis and hepatocellular carcinoma, and also provide guidance to medical staff in gastroenterology at Hokkaido University. Since 2017, I have been the principal researcher of the Japan Medical Research and Development, Research Project for Practical Use of Overcoming Hepatitis, etc. “Analysis of Tissue Pathogenesis Mechanism of Chronic Liver Disease and Comprehensive Analysis for Glycans of Serum and Tissue”
Glycobiomarkers focus on structural changes of glycans found in specific glycoproteins, which are linked to the onset or severity of various diseases. The technology to analyze glycans and to detect minor structural changes in glycans has made possible a new clinical test involving glycobiomarkers. This article introduces the current status of comprehensive analysis of glycobiomarkers for liver disease, and their use in analysis of glycans in fatty liver disease by our group.
Biopolymers containing glycosylation are known as complex carbohydrates, and include glycoproteins, glycolipids, and proteoglycans. Protein glycosylations are roughly classified into N-Glycans and O-Glycans. Complex carbohydrates are synthesized in all cells in our body, and all cell surfaces are covered with complex carbohydrates. The functions of glycosylation are diverse and hydrophilic, and therefore protein glycosylations are easily soluble in body fluids such as serum. Most of the proteins in the serum are secreted from the liver, but most proteins other than albumin are glycoproteins, and the proteins on the cell membrane are also glycoproteins linked with carbohydrate chains. Glycosylation is the most frequent and important post-translational modification of proteins and has been implicated in many important pathological stages, such as carcinogenesis 1. Glycosylations have very complex structures due to their bonding positions, branched structures, and stereoisomerism. Recently, advances in analysis methods have made it possible to detect this complex carbohydrate chain structure. Therefore, glycosylation analysis related to liver disease is in progress.
Glycomics is the analysis of sugars or glycans, either free or attached to larger molecules such as proteins or lipids. Recently, Mac-2 binding protein glycolysis isomer (M2BPGi) has been developed in Japan as a marker for liver fibrosis 2. M2BPGi is useful for enclosure of advanced liver fibrosis, although the cutoff value varies depending on the background liver disease. Currently, the association with carcinogenesis has also been reported and has already been used clinically.
In this paper, we describe the comprehensive analysis of glycobiomarkers related to liver disease, focusing on hepatocellular carcinoma and non-alcoholic fatty liver disease.
Hepatocellular carcinoma (HCC) is the most important complication of viral and other chronic liver diseases, and the carcinogenesis and the progression of carcinoma greatly affect the prognosis. Fucosylated AFP is known as a glycobiomarker associated with HCC. It is known that the serum AFP concentration levels also increase in benign liver diseases such as acute hepatitis and cirrhosis. Fucosylated AFP has high specificity for detection of HCC as compared with AFP alone 3,4, and has already been used in screening for HCC in chronic liver disease.
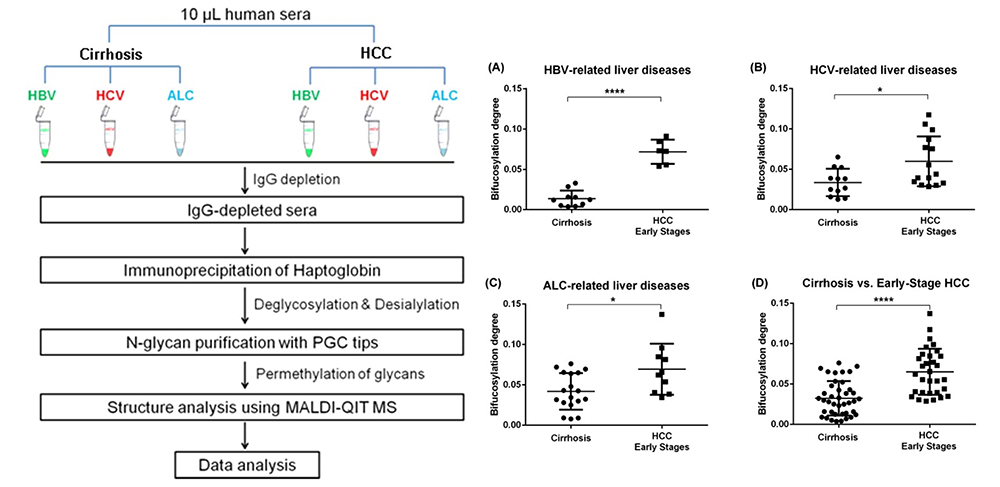
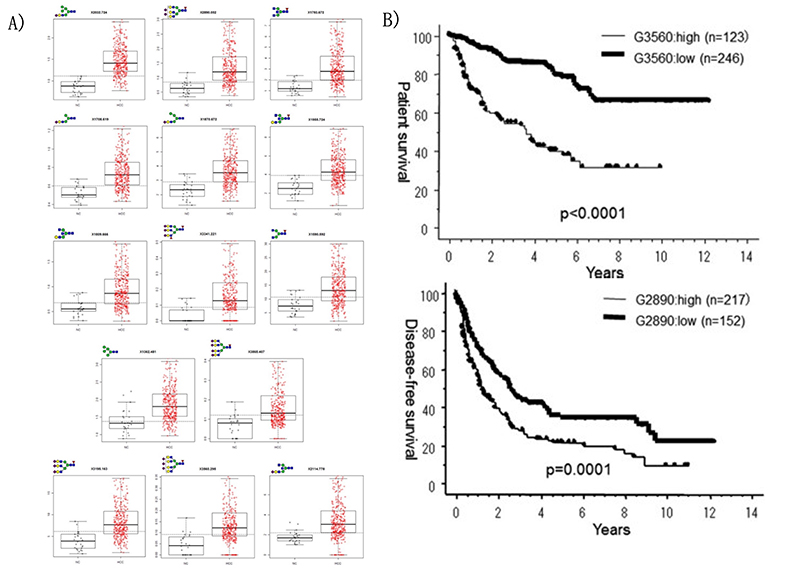
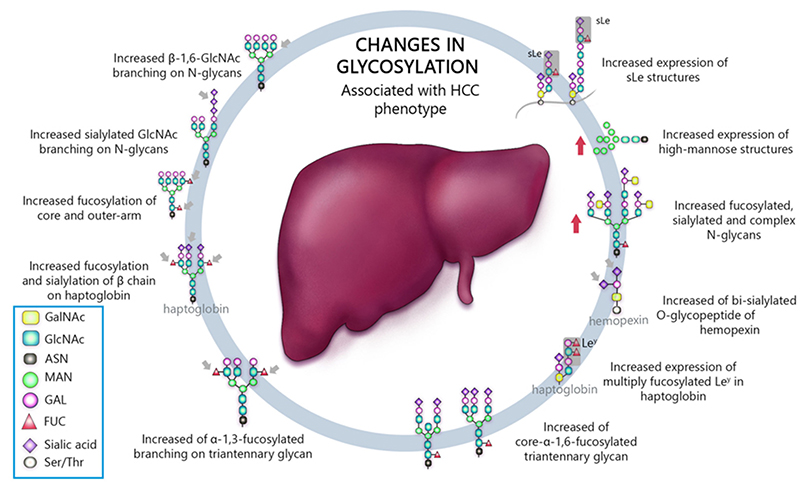
Matrix-assisted laser desorption / ionization mass spectrometry (MALDI-MS) is an effective technique for N-glyconic analysis of clinical samples and is widely used for the analysis of N-glycans in HCC5. Zhu J et al. investigated N-glycans in serum haptoglobin in patients with hepatocellular carcinoma and liver cirrhosis. Haptoglobin obtained by immunoprecipitation was analyzed by MALDI-MS, and it was reported that patients with early hepatocellular carcinoma of each etiological category could be distinguished from cirrhosis by increasing bifucosylation of haptoglobin (Fig. 1) 6. Kamiyama et al. analyzed comprehensive glycosylation on N-glycans using mass spectrometry (MS) from preoperative serum in resected hepatocellular carcinoma cases. A comparative analysis of 369 HCC resected patients and 26 liver transplant donors as a control was performed, and 14 N-glycans were identified as glycosylation significantly altered in HCC cases. Among them, G2890 was identified as a glycobiomarker associated with recurrence, and G3560 as a glycobiomarker associated with prognosis, and these glycobiomarkers were found to be strongly correlated with tumor number, size, and vascular invasion (Fig. 2) 7. Recently, research of glycobiomarkers related to carcinogenesis or biological malignancy of HCC has been in progress, with advances in glycosylation analysis methods 8,9 (Fig. 3).
Chronic viral liver disease is being overcome through the dramatic advances in antiviral therapy for HCV and HBV. The number of HCC cases caused by viral hepatitis tends to decrease year by year, but those caused by fatty liver disease tend to increase. Especially, nonalcoholic fatty liver disease (NAFLD) caused by obesity or diabetes has attracted attention in recent years. NAFLD is sub-divided into non-alcoholic fatty liver (NAFL; a non-progressive form of NAFLD) and non-alcoholic steatohepatitis (NASH; a progressive form that can lead to cirrhosis and the development of hepatocellular carcinoma [HCC])7). To date, liver biopsy remains the gold standard for NAFLD diagnosis and staging. However, liver biopsy also has problems such as sampling error and difference in diagnoses among pathologists. In addition, it is difficult to perform liver biopsy in all NAFLD patients because of invasiveness, and noninvasive biomarkers are expected. However, there are few reports of glycobiomarkers related to NAFLD.
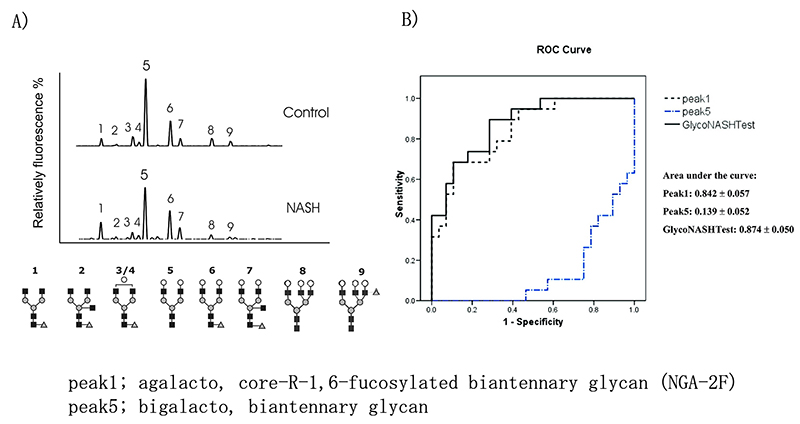
Chen C et al. 10 analyzed the N-glycans of 47 NAFLD patients (NASH 38, NAFL 9) and 13 healthy individuals with DNA sequencer-assisted fluorophore-assisted carbohydrate electrophoresis (DSA-FACE). In NASH patients, agalacto, core-α-1,6-fucosylated biantennary glycan (NGA-2F) is significantly higher, and bigalacto, biantennary glycan (NA2) is significantly lower (Fig. 4A), and this log ratio (GlycoNashTest: log[NGA2F]/[NA2]) was reported to be useful for NASH diagnosis. This GlycoNashTest significantly correlated with liver fibrosis in NAFLD, and the AUC of NASH diagnosis with advanced fibrosis (F3/4) by ROC analysis was 0.874, sensitivity 89.5%, specificity 71.4% (Fig. 4B). Furthermore, Blomme B reported that this glycobiomarker was useful for NASH diagnosis in NAFLD patients scheduled for bariatric surgery 11 and childhood NAFLD patients 12, and showed correlation with pathological lobular inflammation in liver biopsy.
M2BPGi was first demonstrated to be useful for diagnosing liver fibrosis in hepatitis C, and has been reported to be associated with liver fibrosis in various diseases. In NAFLD, the usefulness of M2BPGi for diagnosis of fibrosis 13 and prediction of HCC 14 has been reported. Kamada et al.15 analyzed the usefulness of the same glycobiomarker, fucosylated haptoglobin, for the diagnosis of NAFLD. Furthermore, they reported that NASH diagnosis was further improved by combining fucosylated haptoglobin and M2BP.
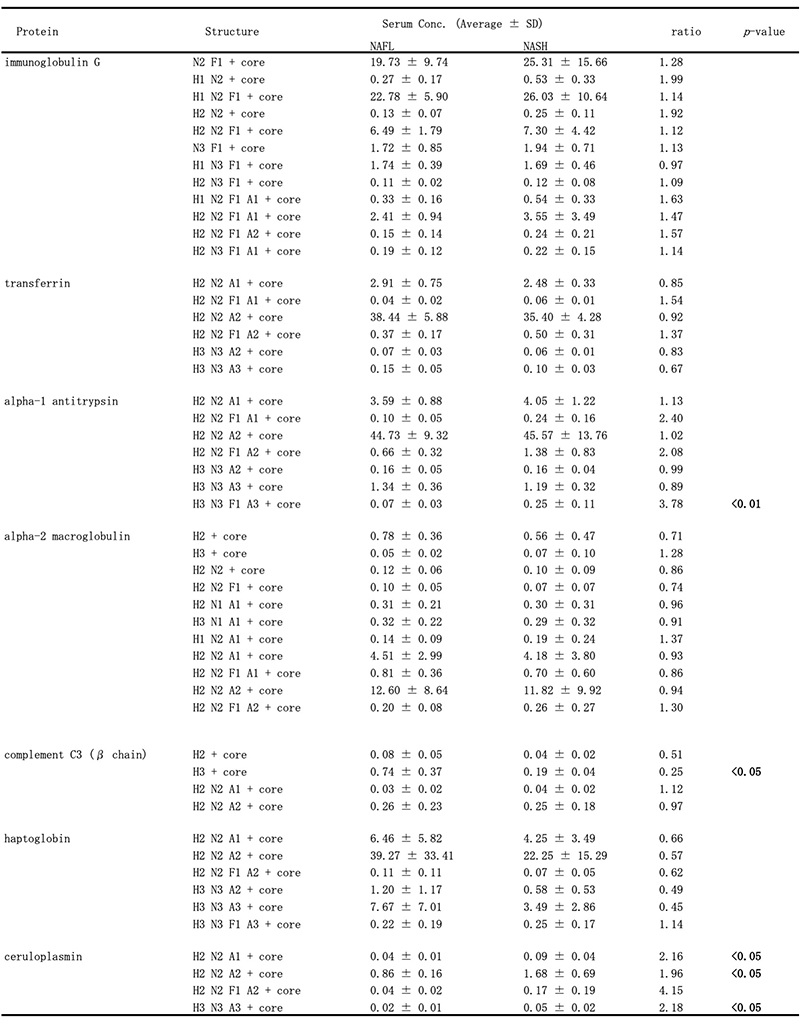
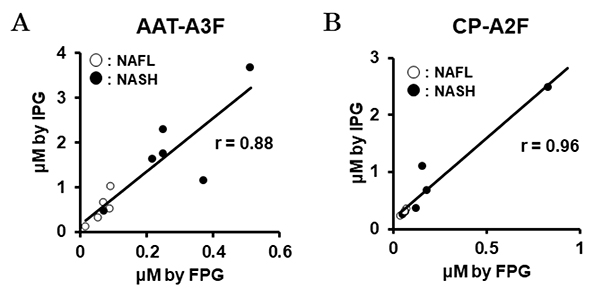
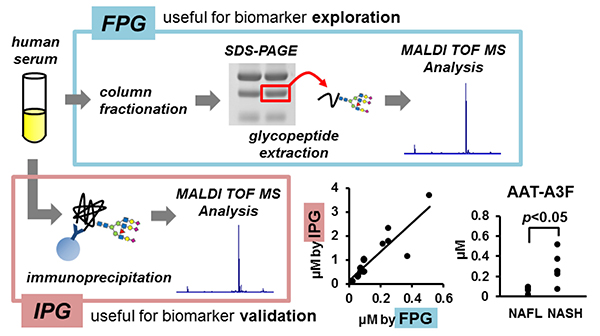
Recently, we have also conducted comprehensive analysis of glycobiomarkers for NAFLD patients. Serum N-glycans have been reported to be potential diagnostic and therapeutic biomarkers for many diseases and conditions, such as inflammation, fibrosis, and cancer progression. In NAFLD, some glycans have been reported that can be biomarkers for NASH diagnosis 16. Therefore, we analyzed N-glycans in NAFLD patients using focused protein glycomics (FPG method) 17. With this methodology, we sought novel glycan biomarkers for nonalcoholic steatohepatitis (NASH) and successfully identified some N-glycans that were significantly elevated in NASH patients compared to nonalcoholic fatty liver patients. Among them, trisialylated monofucosylated triantennary glycan of alpha1 antitrypsin (AAT-A3F) showed the most dynamic change and was considered the most useful biomarker (Table 1). However, although the FPG method is useful for comprehensive analysis, it is not suitable for examination in many cases because it is complicated and time-consuming. Therefore, we developed an immunoprecipitation-glycomics (IPG) method that enables analysis in many cases of target glycosylations 18. We compared the values of N-glycans determined by FPG and IPG. The quantified values of each N-glycan by these two methods showed a statistically significant correlation, indicating that high throughput and quantitative N-glycomics of targeted proteins can be achieved using a simplified IPG method (Fig. 5). Thus, FPG method is useful for comprehensive analysis to extract disease-related glycans from a number of glycan structures, and when used in combination with IPG method to verify the clinical usefulness of the extracted target glycans, it becomes possible to analyze glycosylation related to diseases such as liver disease (Fig. 6) 19. To verify the usefulness of AAT-A3F, which has the most difference between NASH and NAFL, we are conducting verification research in cooperation with multiple institutions.
In clinical practice of liver disease, noninvasive biomarkers are needed depending on the pathological condition such as liver fibrosis, inflammation, and hepatocellular carcinoma. In particular, NAFLD is expected to occupy an important position in liver diseases as the hepatitis virus is overcome. Therefore, glycosylation related to NAFLD is considered to be a field that will receive more attention in the future. It is expected that elucidation will progress along with the progress of analysis methods of glycomics.