
Kimie Date
Project Lecturer, Institute for Human Life Innovation, Ochanomizu University
2002: Researcher, Fuji Gotemba Research Laboratories, Chugai Pharmaceutical Co., Ltd.
2005: Patent scientist, Sawaga international patent office
2012: PhD, Biochemistry, Ochanomizu University (Laboratory of Prof. Haruko Ogawa)
2010: Research Fellowship for Young Scientists (DC2 and PD), the Japan Society for the Promotion of Science (JSPS)
2015: Restart Postdoctoral Fellowship (RPD), JSPS
2016: Research fellow, Yale University
2017: Institute for Human Life Innovation, Ochanomizu University, Project Lecturer Dr. Date investigates the functions of the carbohydrate-binding activity of pancreatic enzymes and glycosylation of extracellular matrix glycoprotein.
Amylase was the first enzyme discovered, in 1833. The enzyme digests to malto-oligosaccharides or maltose from polysaccharides such as starch, and development of modified substrates and inhibitors has continued since the enzyme was discovered. Pancreatic α-amylase is synthesized by pancreas and secreted into small intestine to digest polysaccharides. We have been conducting research from a slightly different perspective than previous amylase studies as digestive enzymes, triggered by the discovery that pancreatic α-amylase binds to the N-glycan of glycoproteins. Recently, previously unknown functions of pancreatic α-amylase other than polysaccharide digestion have been discovered, such as that it determines the localization of pancreatic α-amylase in the small intestine and regulates carbohydrate digestion and absorption due to its carbohydrate-binding activity. We introduce these discoveries below.
Pancreatic enzymes are mainly composed of digestive enzymes that degrade polysaccharides, lipids, proteins, and nucleic acids. There are four types of lipolytic enzymes, nine types of proteolytic enzymes, and two types of nucleolytic enzymes. Thus, digestion is complemented by the presence of a plurality of enzymes for each substrate. However, the only pancreatic digestive enzyme for polysaccharides is α-amylase 1-3. Proteases are synthesized as inactive proenzymes in pancreatic acinar cells, whereas other pancreatic enzymes, including α-amylase, are synthesized as active forms. α-Amylase comprises 26.5% of rat pancreatic juice, which has the highest content of pancreatic enzymes 2. In the process of researching plant lectins, we discovered that pancreatic α-amylase has carbohydrate-binding activity. We are currently elucidating the biological significance of the newly discovered activity of the α-amylase. Our research aims to inform further studies that may lead to dietary therapies and drug discovery that can help prevent and treat diabetes and obesity, and improve the symptoms of carbohydrate digestive abnormalities.
α-Amylase [EC 3.2.1.1] is an endo-type enzyme that hydrolyzes α-1,4 glucose linkages of polysaccharides, and produces large amounts of maltose. Although human α-amylase is detected in organs other than the pancreas and salivary glands, most of the α-amylase present is derived from the pancreas and salivary glands. The amino acid compositions of both pancreatic and salivary amylases have 97% similarity, and both have two N-glycosylation sites at the C-terminus. Pancreatic α-amylase, molecular weight 54,000, has almost no sugar chain. Salivary gland α-amylase is categorized as family A or B, indicating its structure as with or without glycosylation, with molecular weights of 62,000 and 56,000, respectively 4,5. Our carbohydrate analysis by HPLC also confirmed that the porcine pancreatic α-amylase is hardly glycosylated. It is reported that glycosylation of α-amylase does not affect substrate affinity or pancreatic exocrine secretion, while the glycosylation improves thermal stability 4,5. α-Amylases are also present in the blood, and these amylases are derived from the pancreas and saliva in a ratio of approximately 1:1 6. The ratio of these amylases and their activity in the blood is used for diagnosis of pathological conditions such as pancreatitis. However, the role of α-amylase in blood and its route to blood from pancreas have not been clarified yet.
The carbohydrate-binding specificity of pancreatic α-amylase was first examined for porcine pancreatic α-amylase (PPA) by sugar elution using a glycoprotein column immobilized on Sepharose beads, ELISA using sugar biotinyl polymer probes, and surface plasmon resonance analysis 7. PPA binds most strongly to mannose at the monosaccharide level. For glycoproteins, PPA binds well to glycoproteins with N-glycans, but does not bind to mucin-type O-glycans (Fig. 1). The binding of PPA to fetuin and transferrin, which have mainly complex N-glycans, is weak at neutral pH and becomes stronger at acidic pH. On the other hand, PPA binding to ribonuclease B, having a high-mannose N-glycans, binds not only under weak acidity but also under neutral conditions. In addition, recombinant human pancreatic α-amylase (recHPA) was expressed in yeast and purified to confirm it as the amylase from humans and not from pig. It has been confirmed that recHPA has the same carbohydrate-binding specificity as PPA 8. The association constants Ka of PPA to glycoproteins are on the order of 10 6-7 M -1, which is about 1-2 orders of magnitude lower than Ka of plant lectin. The small intestine, where pancreatic α-amylase works as a digestive enzyme for polysaccharides, is covered with a sugar chain matrix called glycocalyx. Although the main component is mucin, many membrane glycoproteins with N-glycans are expressed in the small intestinal brush border membrane (BBM). It has been found that pancreatic α-amylase interacts with the N-glycan of membrane glycoproteins on the small intestinal BBM due to its carbohydrate-binding activity. These characteristics are illustrated below.
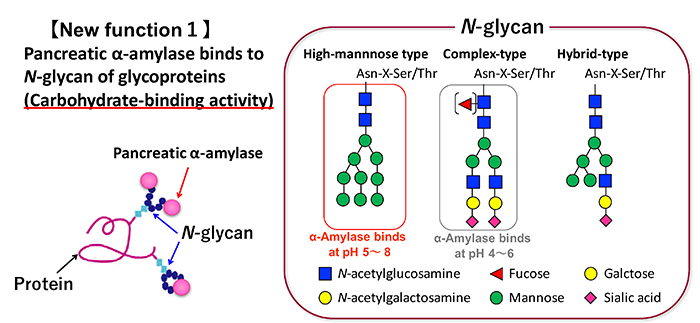
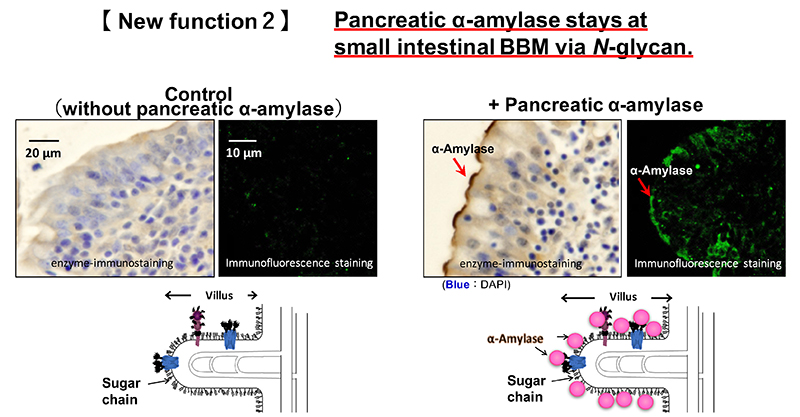
Pancreatic α-amylase is synthesized in pancreatic acinar cells and packed along with other digestive enzymes in zymogen granules. These digestive enzymes in the zymogen granules are released by exocytosis with neurohumoral stimulation such as gastrointestinal hormone 9. These enzymes are then delivered into the duodenum from pancreatic duct. The localization of pancreatic α-amylase after reaching the duodenum has not been elucidated. We investigated the relationship between pancreatic α-amylase localization and its carbohydrate-binding activity in the duodenum. Immunohistochemical staining using porcine duodenum revealed that pancreatic α-amylase binds to the BBM of the duodenum 8. The binding of pancreatic α-amylase to the duodenal BBM is inhibited by mannan, which is the polysaccharide of mannose, but not galactan or colominic acid, which are the polysaccharides of galactose and N-acetylneuraminic acid, respectively. Both these results and the carbohydrate-binding specificity of pancreatic α-amylase suggest that pancreatic α-amylase binds to high-mannose N-glycans on the small intestinal BBM (Fig. 2). The effects of the pancreatic α-amylase binding to the small intestinal BBM on the digestion and absorption of carbohydrates was examined. The results are illustrated below.
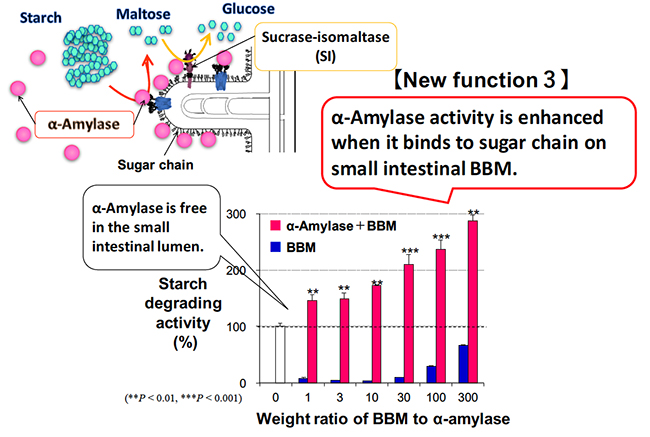
Dietary polysaccharides are digested to disaccharide maltose by α-amylase in the pancreatic juice in the duodenum. The maltose is broken down to monosaccharide glucose by sucrase-isomaltase (SI), which is a membrane enzyme on the small intestinal BBM. Maltase-glucoamylase is also expressed in the small intestinal BBM, and breaks down maltose into glucose. The contribution rate of enzymes that break down maltose in the intestine is reported to be 80% for SI and 20% for maltase-glucoamylase 10,11. In the duodenal lumen, pancreatic α-amylase is responsible for digestion from polysaccharides such as starch to maltose, and mainly sucrase and isomaltase are responsible for digestion from maltose to glucose. In this way, pancreatic α-amylase and SI cooperate to perform carbohydrate digestion. We focused on the cooperative digestion, and examined the interaction between pancreatic α-amylase and the SI on the small intestinal BBM.
As described above, when pancreatic α-amylase is secreted from the pancreas and reaches the duodenum, it binds to the N-glycans on the small intestinal BBM due to its carbohydrate-binding activity. Pancreatic α-amylase binds to the small intestinal BBM, which increases enzymatic activity to about three times higher compared to when it does not bind (Fig. 3). SI forms a complex with sucrase and isomaltase; there is a transmembrane site on the sucrase side, and isomaltase protrudes to the small intestinal lumen side. SI is composed of highly glycosylated glycoproteins having complex and high-mannose N-glycans and O-glycans, and has molecular weight of 215 kDa 12,13. Pancreatic α-amylase binds to an N-glycans of SI containing mannose on the small intestine BBM. This interaction enhances the maltose degradation activity of SI by about two-fold 8. Pancreatic α-amylase bound to the BBM also has enhanced starch-degrading activity as described above. Thus, the carbohydrate-binding activity of pancreatic α-amylase facilitates the cooperative digestion from starch to glucose in the small intestine by interacting with the N-glycans of SI, resulting in efficient energy acquisition.
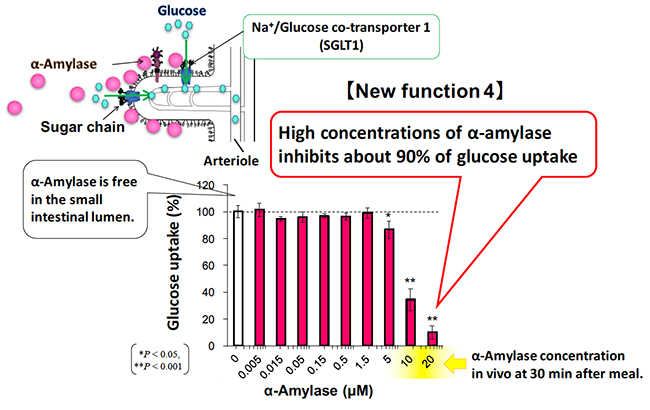
There are two kinds of glucose transporters in our body, GLUT (glucose transporter) and SGLT (sodium / glucose cotransporter). Glucose absorption in the small intestine is actively performed by SGLT1 expressed in the BBM of intestinal epithelial cells. Glucose taken up into cells is passively transported by GLUT2 expressed in the basolateral membrane, resulting in an increase in blood glucose level 14. SGLT1, expressed in the rabbit small intestine, has a tri- and tetra-antennary complex N-glycans in the extracellular domain 15. Since there has been no report regarding the relationship between pancreatic α-amylase and SGLT1, we examined the effect of pancreatic α-amylase on glucose uptake of SGLT1, using reconstituted BBM vesicles.
When the sodium-dependent glucose uptake of SGLT1 was measured in the presence or absence of the α-amylase in the small intestinal BBM vesicle, pancreatic α-amylase inhibited glucose uptake of SGLT1 in an α-amylase concentration-dependent manner 8. The glucose uptake of SGLT1 was inhibited by about 90% under high concentration of pancreatic α-amylase (Fig. 4). Protein concentration of pancreatic juice is 4.2 mg/ml in normal pigs and 5-16 mg/ml in normal cows 16. Because pancreatic α-amylase comprises about 26.5% of total protein in the pancreatic juice, pancreatic α-amylase concentrations that inhibit SGLT1 activity are considered to be physiologically possible.
Research and development of SGLT inhibitors as oral anti-hyperglycemic agents is underway. Phlorizin in apple and pear bark is known to lower plasma blood glucose levels and inhibits SGLT 17. However, an effective blood concentration of phlorizin is difficult to maintain, because of metabolization by oral administration. For this reason, pharmaceutical companies have developed SGLT inhibitors targeting metabolically stable compounds. SGLT2 inhibitors selectively inhibit only the SGLT2 that is specifically expressed in renal tubules, and promote excretion of excess glucose through the urine. Use of SGLT inhibitors is regarded as an innovative treatment for diabetes with low risk of hypoglycemia 18. It is reported that an oral drug inhibiting both SGLT1 and SGLT2 has also been shown to be effective for treatment of diabetes mellitus in recent years 19.
As described above, pancreatic α-amylase binds to N-glycans on the BBM of small intestine, thereby enhancing polysaccharide digestion while suppressing glucose absorption. Pancreatic α-amylase is clearly stained with immunofluorescence staining in non-fasted pig small intestine, whereas it is hardly stained in fasted pig small intestine. Therefore, pancreatic α-amylase was added to a fasted pig small intestine, and the localization was observed. The results showed that pancreatic α-amylase bound to the BBM at 4°C and gradually moved into the tissue at 37°C, and eventually localized to lysosomes 20. Another experiment, using differentiated Caco-2 intestinal epithelial cells, revealed that pancreatic α-amylase bound to the cell membrane at 4°C and was internalized to the lysosome via the endocytosis pathway at 37°C. Because pancreatic α-amylase is not completely degraded by lysosomes, there is a possibility that it may be internalized into small intestinal epithelial cells and then exocytosed into the blood.
SGLT1 on the small intestinal BBM co-localizes with pancreatic α-amylase 20. However, it is not clear whether SGLT1 is endocytosed with pancreatic α-amylase, stays in the membrane, or moves via a different pathway than pancreatic α-amylase. Experiments using differentiated Caco-2 cells revealed that SGLT1 localized on the cell surface disappeared within 30 minutes after the medium change and returned to the cell surface again within 60 minutes 20. SGLT1 has a half-life of 2.5 days 21; therefore, SGLT1 is thought to be recycled on and off the cell surface. The SGLT1 recycling is unaffected by exogenous pancreatic α-amylase and can be observed only by changing phosphate buffer or medium. The recycling is also unaffected by the lysosomal inhibitor chloroquine. From these results, SGLT1 may be endocytosed from the membrane into the cell by stimulation similar to medium exchange, such as gastric or pancreatic juice flowing onto the BBM of the small intestine. This could be a self-defense function that prevents a sudden increase in blood glucose level. Interestingly, the blood glucose level is highest 30 minutes after a meal, and SGLT1 is endocytosed 30 minutes after a medium change: both times are consistent 20.
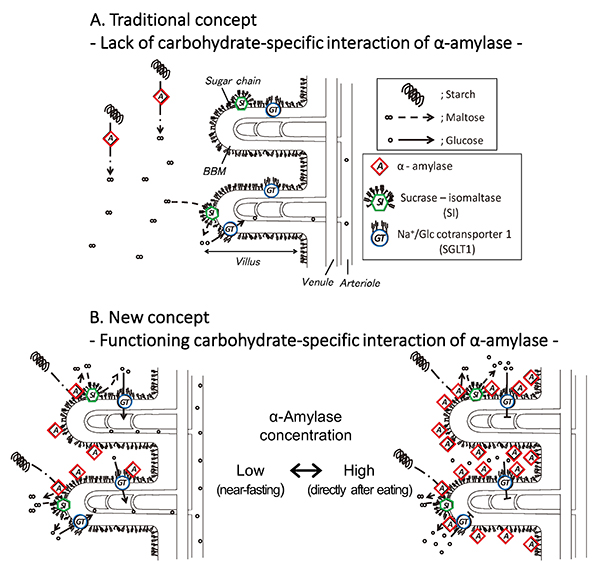
In the traditional concept, in which α-amylase is considered to lack a carbohydrate-specific interaction, pancreatic α-amylase remains apart from brush border membrane (BBM), and digests starch flowing in the small intestinal lumen. Sucrase-isomaltase (SI) also digests only nearby maltose into glucose (Fig. 5A). The discovery of the carbohydrate-binding activity of pancreatic α-amylase suggests the following new concept for glucose assimilation (Fig. 5B): Pancreatic α-amylase secreted from the pancreas to the duodenum remains bound to the N-glycans on the small intestinal BBM, where SI and SGLT1 are expressed. Both the α-amylase and SI are activated and exist in close proximity to work cooperatively in degrading starch to produce glucose. The glucose is incorporated into enterocytes via SGLT1 under low concentration of α-amylase (Fig. 5B, left). The α-amylase inhibits the glucose absorption of SGLT1 under high concentration of α-amylase, which will occur for a short time after secretion of pancreatic fluid (Fig. 5B, right). Therefore, the carbohydrate-binding activity of pancreatic α-amylase has one function to enhance starch digestion together with SI so that hypoglycemia does not occur under fasting condition, and another function to inhibit SGLT1 thereby preventing a rapid increase in blood glucose level after eating (postprandial hyperglycemia). It is thought that the α-amylase contributes to blood glucose level homeostasis by selectively using these two contradictory functions, depending on the concentration.
As a result of the discovery of the carbohydrate-binding activity of pancreatic α-amylase, it was shown that the α-amylase has functions of regulating membrane digestion and glucose absorption in addition to its function as a digestive enzyme that degrades polysaccharides. We hope to discover agents that regulate the digestion and absorption of carbohydrates by promoting or inhibiting the interaction between pancreatic α-amylase and N-glycans on the SI and SGLT1, and can be used for the prevention and treatment of diabetes mellitus and obesity.
Acknowledgment
The results presented here were obtained with the cooperation and support of many people. This research is based on the research started by Dr. Haruko Ogawa of Ochanomizu University. I sincerely thank Dr. Haruko Ogawa. I sincerely thank Dr. Yoshifumi Jigami, Dr. Ken-ichi Nakayama, and Dr. Mariko Umemura of the National Institute of Advanced Industrial Science and Technology for expression of recHPA in yeast, and thank Dr. Ayano Sato of Okayama University for culture of Caco-2 cells. I thank Dr. Yoshifumi Jigami again, for support through the Dr. Yoshifumi Jigami Memorial Fund (The Society of Yeast Scientists).