
Kyoko SHINYA
Associate Professor: Division of Zoonosis, Department of Microbiology and Infection, Graduate School of Medicine, Kobe University.
Short CV: Ph.D.; Yamaguchi University, Research Associate (2004-2005); Tohoku University, Associate Professor (2005-2007); Tottori University. Research subjects: Pathogenesis of influenza virus infection, Fidelity of influenza virus polymerase.

Yasuhiro SUZUKI
Associate Professor: Department of Complex Systems Science, Graduate School of Information Science, Nagoya University.
Short CV: Ph.D.; Kyoto University, Research Associate (1997-2004); Tokyo Medical and Dental University, Visiting Scientist (2003-2004); ATR Laboratories. Research subjects: Science of Complex Systems, Systems Biology, Natural Computing.
Glycans in living creatures exist mainly as complex glycans, such as proteoglycans, glycoproteins and glycolipids. Proteoglycans are characterized as having extended sugar chains linked to a core protein. These chains are formed by two repeating monosaccharides and are one of the most common components of the extracellular matrix in animal tissue. Glycoproteins consist of a core protein and several monosaccharide chains. In glycoproteins, these sugar chains link to the core protein at either the aspartic acid (in the case of N-linked sugar chains) or the serine/threonine (in the case of O-linked sugar chains). Glycolipids are classified into two groups: sphingoglycolipids and glyceroglycolipids. Sphingoglycolipids consist of a ceramide and monosaccharides. They are mainly classified as cerebrosides and gangliosides. Glyceroglycolipids are glycolipids containing more than a single glycerol molecule. They are found in some types of animal cells, but more commonly in plant cells and bacteria.
The glycans located on the surface of the cell membrane are one component in the construction of glycoproteins and glycolipids. These glycans are linked to the core proteins and core lipids produced by the cell and inserted into its membrane. They are an important functional molecule in all animal tissue. Such complex glycans cover the entire cell and are continually renewed. They are also found in the functional microdomains related to signal transduction and endocytosis in the double-layered lipid membrane.
Among the monosaccharides of which these membrane-associated glycans are constructed, sialic acids are those located at the ends of glycans and therefore directly involved in the recognition and control of a variety of both internal and external molecules. Sialic acids exhibit a remarkable variety of structures: the molecular changes within sialic acids located on the surface of mucosa or immune cells and the variation in how the internal molecules of different animal species recognize them are considered to be the results of species' evolution 1-3.
1) Influenza virus-derived lectins.
“Lectin” is the general term used to describe a glycan-recognizing molecule. In addition to internal lectins, which play a role in development and differentiation as well as in the maintenance of homeostasis in animals, there are many external lectins derived from bacteria, viruses and plants.
In the case of influenza viruses, two types of lectins, hemagglutinin (HA) and neuraminidase (NA), exist on the surface of virus particles. Because these two molecules recognize and bind to the sialic acids of the surface of animal cells, the influenza HAs and NAs are categorized as external lectins in animals. Influenza A viruses are referred to as “zoonotic microorganisms”, and depending on the type of sialic acid found on the host animal’s cell membranes, structural changes and/or modifications occur in their HA and NA molecules. For example, avian and human-derived influenza A viral HAs preferentially bind to either the avian-type α2,3-linked sialoglycans, or to the human-type α2,6-linked sialoglycans, depending on the sialic acid of the host 4.
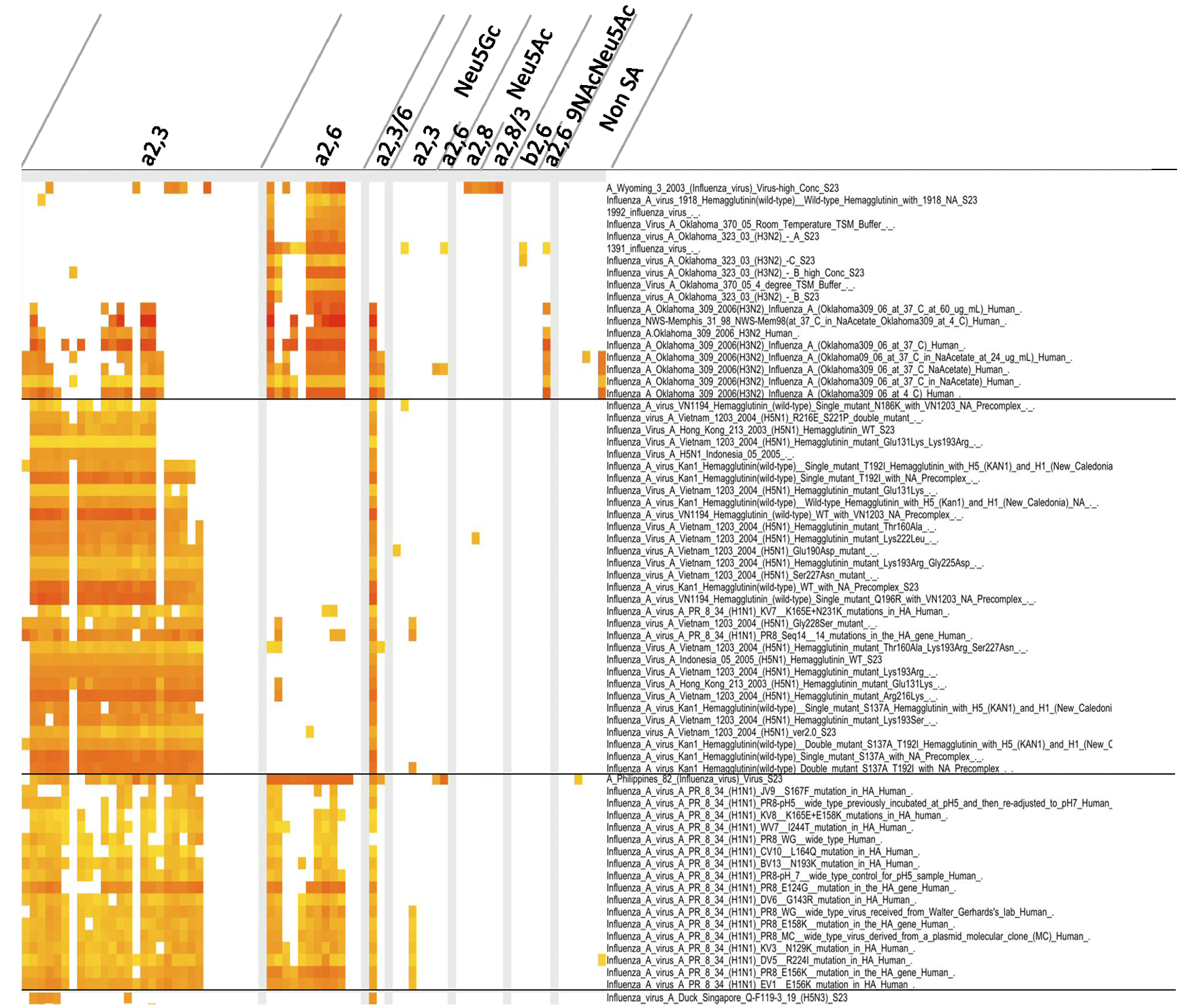
Figure 1 Glycan binding properties of influenza viruses.
We collected glycan array data from the CFG database. The binding properties were analyzed using the ODANGO system. In brief, the lectin-glycan binding properties were divided into two groups (weak and strong). The weak group exhibited the normal (or Gaussian) distribution pattern, with those of the strong group outliers of the normal distribution. We therefore defined these outliers to be “specific” lectin-glycan bindings, and the specific bindings defined as positive. The list of glycans appears in the link (http://dl.dropbox.com/u/23655125/Shinya_et_al_Table1_glycan-list.xlsx). The ODANGO system was developed by Suzuki Laboratory, Department of Complex Systems Science, Graduate School of Information Science, Nagoya University.
In our analysis, we utilized the glycan array data of the Consortium for Functional Glycomics (CFG) and an interactive CFG viewer “ODANGO ver.1” in order to conduct a pattern analysis of lectin-glycan bindings (Figure 1). The results showed that the glycan binding pattern of influenza viruses varied with different virus strains. A human-derived influenza A virus isolated in 2003 showed a propensity for binding with α2,6-linked sialoglycans, except in high concentrations. Such glycan binding is typical for human-derived viruses 4. However, there are exceptions, such as the case of a virus isolated in 2006, where the human-derived influenza A virus was found to be able to recognize both α2,3-linked sialoglycans and α2,6-linked sialoglycans. The human-derived H5 viruses and their mutants generally recognized α2,3-linked sialoglycans, although several mutants were observed to bind with a few α2,6-linked sialoglycans. A vaccine strain, A/Philippines/2/82 (H3N2), and a laboratory strain, A/Puerto Rico/8/34 (H1N1), are human-derived strains, but recognized both α2,3-linked sialoglycans and α2,6-linked sialoglycans. These kinds of glycan-binding properties may be a reflection of their history of being passaged in embryonated chicken eggs. A duck-derived strain, A/Duck/Sigapore/Q-F119-3/19 (H5N3), also recognized α2,3-linked sialoglycans.
These findings indicate that some influenza virus-derived lectins have an affinity with a wide range of sialoglycans.
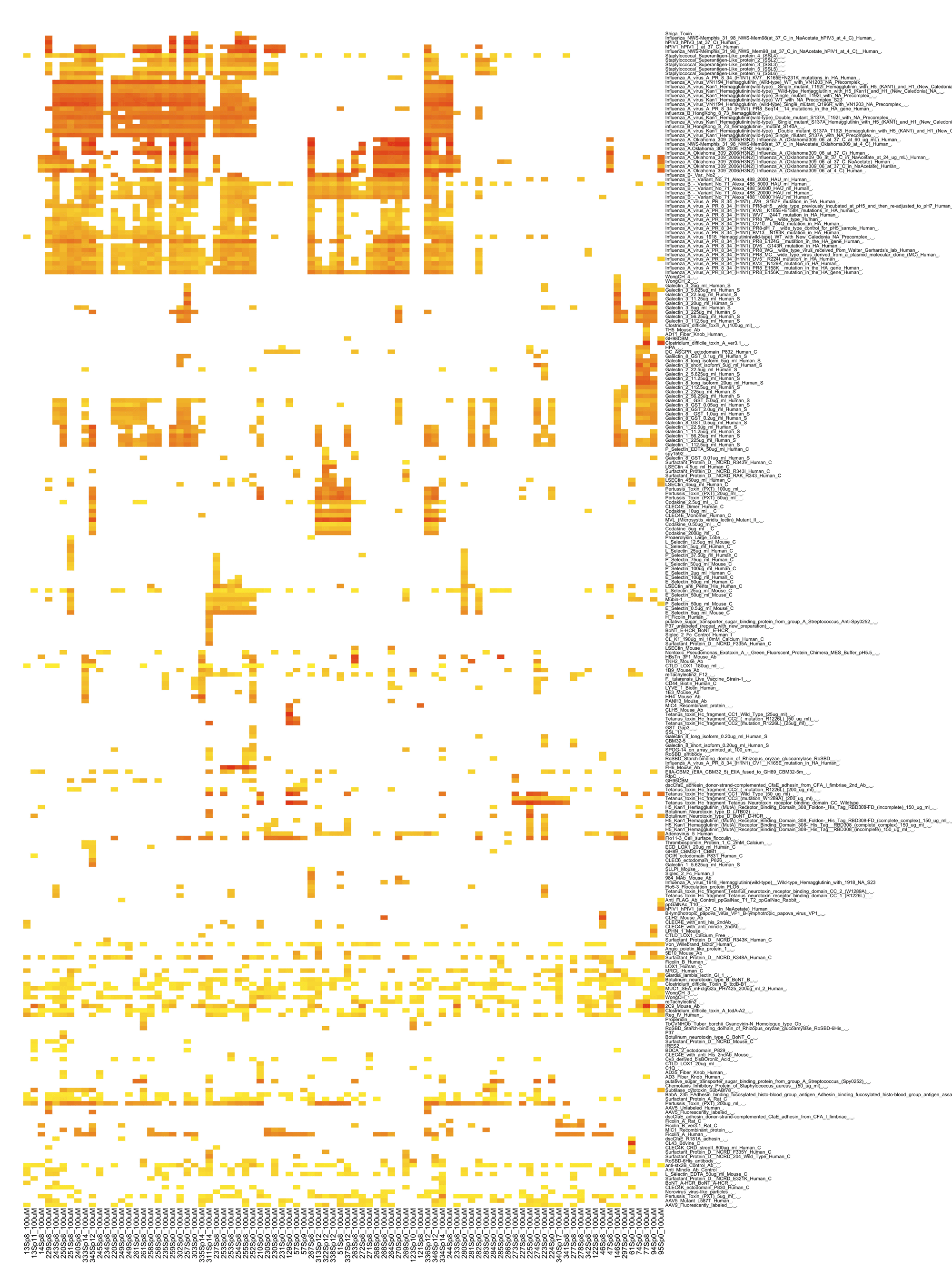
Figure 2 The glycan recognition pattern of the influlenza virus-derived lectins and internal lectins.
Glycan array data for a variety of lectins has been collected from the CFG database and analyzed as described in the legend for Figure 1.
2) Differences in glycan recognition between influenza virus-derived lectins and internal lectins.
After achieving entry into the host, influenza viruses replicate in situ, producing the influenza virus-derived lectins HA and NA. However, it was unknown how the appearance of massive amounts of these external lectin molecules affects the host.
To answer this question, the previously described comparative analysis of glycan binding properties using the glycan array data of the CFG and ODANGO system was conducted on both internal lectins and pathogen-derived lectins such as influenza viruses and bacterial toxins (Figure 2). The results revealed that pathogen-derived lectins, such as influenza viruses, adenoviruses, bacterial toxins (tetanus, pertussis, staplylococcal, etc.) showed a wide range of glycan binding. However, most of the internal lectins restricted themselves to binding with only a few glycan structures, with the exception of internal lectins such as galectin 1 and 8. In addition, when the glycan binding properties of internal and external lections were compared, the internal lectins, which bind to a wide range of glycans, showed binding patterns similar to those of influenza A viruses. Invading pathogens are sometimes designated as “homeostasis disruptors” when they cause diseases in their hosts. In this case, the appearance of massive numbers of pathogen-derived lectins, all having glycan-binding patterns similar to those of the host’s own internal lectins may contribute to the disruption of the homeostasis maintained by these internal lectins.
1) Distribution pattern of sialoglycans in hosts.
Many types of sialoglycans are found in host animals. While conducting other influenza virus research, we examined the distribution of α2,3-linked sialoglycans and α2,6-linked sialoglycans in both human respiratory tracts and the intestinal mucosa of waterfowl. The binding patterns of typical plant-derived lectins (Sambucus nigra lectin and Maackia amurensis lectin recognized α2,6-linked sialoglycans and α2,3-linked sialoglycans, respectively) reconfirmed the presence of α2,3-linked sialoglycans on the surface of colon mucosa of waterfowl (unpublished data) 5. In addition, we detected α2,6-linked sialoglycans as well as a small number of α2,3-linked sialoglycans in the tissues of the human nasal respiratory area, α2,6-linked sialoglycans on the surface of tracheal and bronchial mucosa, and α2,3-linked sialoglycans on the Clara cells located between terminal and respiratory bronchioles and type II pneumocytes 6. As mentioned above, HAs of avian-derived influenza viruses preferentially recognize α2,3-linked sialoglycans, while HAs of human-derived influenza viruses prefer the α2,6-linked sialoglycans. Therefore, the distribution of the sialoglycans found in the hosts correlates well with the glycan binding properties of their influenza viral HAs. Furthermore, highly-pathogenic avian influenza viruses, which are more liable to cause severe pneumonia, possess avian-derived virus type HAs. In their reports on such cases, pathologists sometimes found intact or regenerative tracheal/bronchial epithelia during microscopic examination. These findings indicate that the growth of viruses with avian-type HAs in the tracheal/bronchial epithelia of these patients is temporary and inefficient 7. In summary, the glycan-binding specificity of viral HAs reflects the distributions of the host's sialoglycans.
2) Possible target cell changes reflecting changes in HAs glycan binding properties.
It is likely that the binding properties of HAs to glycans affect the distribution of the influenza virus in the respiratory tract. Such a theory is supported strongly by the results of an experiment using pandemic 2009 H1N1 viruses, in which HA mutants able to recognize α2,3-linked sialoglycans increased the infectivity of type II pneumocytes possessing α2,3-linked sialoglycans 8. Similar virilogical studies focusing on the distribution of respiratory sialoglycans show that steady progress is being made in this research area. However, little research specifically focusing on immune cells possessing large numbers of functional sialoglycans on their surface have appeared. Only a few studies have been published examining how changes in the glycan-binding properties of H5 viruses alter immune response 9, 10. In fact, the type of sialoglycans appearing on the immune cells varied with the type of immune cell 11. Further research will be needed in this area.
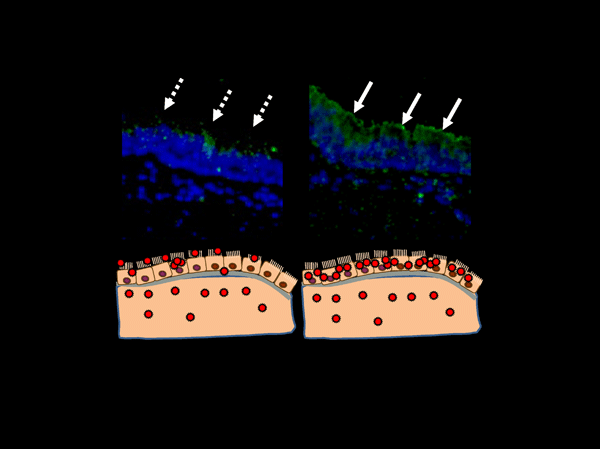
Figure 3 The biological significance of changing the glycan binding properties of influenza A virus HAs.
When the sialoglycan binding property of a viral HA matches the sialoglycan type of the human respiratory mucosa, the virus particles are efficiently attached to the epithelia of the respiratory mucosa (right upper panel). This further concentrates the virus particles on the targeted cells to establish a more effective infection.
Whether they are internal or external, lectins have a weak affinity with sugar chains compared to that of antigens/antibodies 12. The strength of the binding quantities of their molecules compensates for this weakness. Therefore the amount of both lectin and sugar molecules, appearing on the HAs of the virus and the sialoglycans of the host cell, strongly affect their binding efficiency, i.e., if influenza viruses possess HAs with a specific affinity for the sialoglycans of the target host cells, the viral particles will be more efficiently concentrated against the targeted cells and, therefore, more infectious. This could be a significant advantage in enabling their survival. In fact, our previous experiments revealed that influenza viruses with HAs specific to the sialoglycans in a human bronchus were more efficient in their ability to attach to the epithelial cells of the human bronchus 13.
Sialoglycans have been described as the “receptors” for influenza viruses. However, more recent studies have shown viral entry being achieved without the presence of sialoglycans 12,14-16. Moreover, a relatively inefficient viral attachment to the cell was found to be compensated for by the density of the infection. In addition, one part of the CFG datum described an influenza viral HA with unusual glycan binding properties in high concentrations (Figure 1). This suggests that it may be more correct to describe sialoglycans as “a sort of intermediary molecule utilized to concentrate the virus in attaching more efficiently to targeted cells”. A more efficient viral attachment to the target cells would secure infection into the target cells in vivo (Figure 3).
The other influenza virus-derived lectin, NA itself, has an enzymatic function, but the part this lectin plays in pathogenesis remains unknown: an attempt to develop a transgenic mouse which would systemically express the influenza NA molecule was unsuccessful. It is possible that the enzymatic function of NA molecules may inhibit the signal transduction mediated by the sialoglycans during the stages of development. In addition, desialylation of the immune cells, resulting in hyper-reaction against the external stimulation, was observed. These facts reconfirmed the important role of sialoglycans in recognizing and controlling a variety of biological functions. Further research will be needed to elucidate the pathogenic role of this desialylation by the influenza NA expressed in situ.
Acknowledgements
We thank Dr. Yoshihiro Kawaoka of the Institute of Medical Science, University of Tokyo, and Messrs. Kenji Tsunoda and Masahiro Fujie of the Department of Complex Systems Science, Graduate School of Information Science, Nagoya University, for collaborative works. We thank Mr. Bruce Collins for English editing.