James W. Dennis1,2,3
1 Samuel Lunenfeld Research Institute, Mount Sinai Hospital
2 Department of Medical Genetics & Microbiology, University of Toronto
3 Department of Laboratory Medicine and Pathology, University of Toronto
The Golgi N-glycan processing pathway in metazoans generates structural complexity on mature glycoproteins, in large part due to variable numbers of N-acetyllactosamine units. These sequences bind to galectins, a family of soluble N-acetyllactosamine-binding proteins with either 1 or 2 carbohydrate recognition domain (CRD). Galectin affinities for N-glycans are proportional to GlcNAc-branching and the number of N-acetyllactosamine units 1. The highest affinity ligands are tetra-antennary products of β1,6 N-acetylglucosaminyltransferase V (Mgat5) extended with poly N-acetyllactosamine.
The oncogene pathway Ras-Erk-Ets stimulates Mgat5 transcription, and carcinomas commonly display increased β1,6GlcNAc-branching and poly N-acetyllactosamine. Galectin-3 cross-links Mgat5-modified N-glycans on EGF and TGF-β receptors at the cell surface, forming a lattice that delays receptor removal by constitutive endocytosis 2. Galectin-3 has a single CRD and the non-lectin N-terminal domain mediates pentamer formation in the presence of multivalent ligands, and thereby cross-linking glycoproteins in proportion to ligand concentrations 3. Oncogenic signaling down-stream of receptor tyrosine kinases promotes vesicular trafficking and endocytosis, but the concomitant increases in GlcNAc-branching of N-glycans on receptors promotes cross-linking by galectins, and inhibits receptor loss to endocytosis. Therefore the lattice ensures up-regulation of surface cytokine receptors and increased sensitivity to growth factors, in the face of increased membrane and cytoskeletal remodeling which drives cell motility and invasion. Mammary tumor cells from Mgat5-/- display a global loss of sensitivity to cytokines including EGF, IGF-1, PDGF, FGF and TGF-β. This results in suppression of metastasis in vivo 4, and failure of mammary tumors to undergo epithelial-mesenchymal transition (EMT)2. EMT characterizes the invasive phenotype and is dependent on activation of Ras and TGF-β/Smad signaling pathways. Peritoneal macrophages provide another example of a motile and highly endocytic cell that require the galectin lattice. Mgat5-/- macrophages are deficient for cytokine-mediated signaling, phagocytosis, and extravasation in vivo. Galectin-3 deficient macrophages display similar functional defects. The functional redundancy of galectin(s), the diversity of N-glycan ligands and the multiplicity of N-glycan chains per receptor should provide robustness to the lattice model of regulation.。
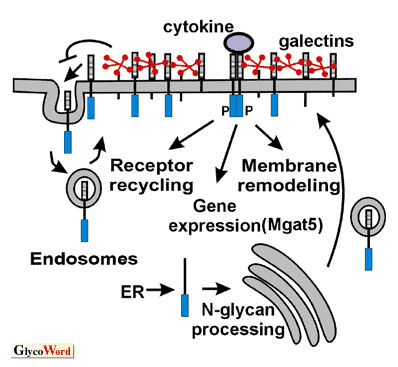
Figure A model of cytokine receptor retention by the galectin lattice.
Activation of Ras/Erk and Smad2/3 pathways stimulates Mgat5 gene expression and modification of surface glycoproteins with β1,6GlcNAc branched N-glycans. These N-glycans on cytokine receptors display increased affinity for galectins; strengthen receptor association with the lattice, thereby oppose constitutive endocytosis and enhance receptor residency time at the cell surface.
It remains to be determined whether regulation of cytokine receptors by galectins is required during embryogenesis. However, it is clear that complex-type N-glycans containing N-acetyllactosamine are required, as their complete absence in Mgat1-deficient mouse embryos is lethal 5. A deficiency in Mgat2 causes reduced N-acetyllactosamine-content in N-glycans, and a postpartum lethality in mice, and morphogenic defects in multiple tissues similar to type II CDG disease in humans. Postpartum lethality is also observed with deficiencies in the major β1,4Gal-T activity (TI), one of 6 genes encoding this activity. Mgat5 is not required for mouse embryogenesis, but adult Mgat5-/- mice display multiple phenotypes including susceptibility to autoimmune disease and suppression of cancer progression; both appear to be galectin-lattice dependent phenotypes 4,6.