
Jianguo Gu
Graduated from the Nantong Medical College, China in 1987. He obtained the PhD degree in 1993 from Department of Biochemistry, Osaka University Medical School (advisor: Prof. Sekiguchi Kiyotoshi). From 1993 to 1997, he worked at Osaka Medical Center for Maternal and Child Health as a researcher (chief: Dr. Yoshinao Wada). He then joined in Dr. Kenneth Yamada's lab in Craniofacial Developmental Biology and Regeneration Branch, NIDCR/NIH as a postdoctoral fellow from 1997 to 1999. In 1999-2002, he was appointed assistant professor, and worked with Prof. Kiyotoshi Sekiguchi at the Institute for Protein Research, Osaka University. He then returned to Prof. Taniguchi's lab (Department of Biochemistry, Osaka University Graduate School of Medicine) in 2002, and was promoted to the rank of associate professor in 2004. His current research interests include the glycobiology of cell adhesion, cancer metastasis, as well as development.
Integrins are cell surface receptors for extracellular matrix (ECM) molecules that are present in all animals and even in the simplest metazoa such as sponges; no homologues have been detected in prokaryotes, plants or fungi1. Integrins consist of α and β subunits. Each subunit has a large extracellular region, a single transmembrane domain and a short cytoplasmic tail (except for β4 integrin). The N-terminal domains of the α and β subunits associate to form the integrin headpiece, which contains the ligand binding site, whereas the C-terminal segments transverse the plasma membrane and mediate interactions with the cytoskeleton and with signaling molecules (Fig. 1A). Based on extensive searches of the human and mouse genomic sequences, 18 α- and 8 β-subunits are known to assemble into 24 integrins including 12 members of the β1 subunit-containing integrins (Fig. 1B). Although many integrins bind to the same ligand or to partially overlapping sets of ligands, each integrin has its own binding specificity. For example, integrin α5β1, a representative integrin, preferentially recognizes fibronectin (FN), a major adhesive component of the matrix, whereas integrin α3β1, a major integrin of epithelial cells or endothelial cells, binds to laminin-5 (LN-5), -10/11 in the basement membrane. Although integrin-mediated adhesion is based on the binding of α and β subunits to a defined peptide sequence, the strength of this binding is modulated by various factors including the status of the glycosylation of integrin. In this brief mini-review, the molecular basis of integrin signaling will be discussed, with the emphasis on roles of N-glycans of α3β1 and α5β1 integrins in their activation and biological functions.
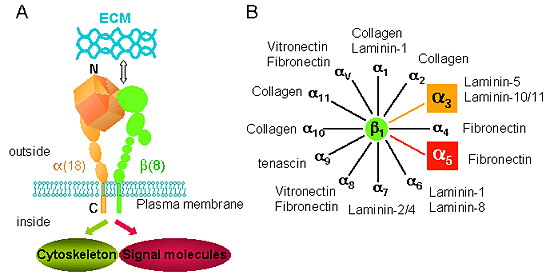
Fig. 1 The integrin receptors and their ligands.
A, Integrins are αβ heterodimers. Each subunit has a large extracellular region and a short cytoplasmic tail (except for β4 integrin). 8 β subunits can associate with 18 α subunits to form 24 distinct integrins. B, Among 24 integrins, 12 members of β1 subunit-containing integrins have been found. They have relative ECM specificities. For example, integrinα5β1 recognizes FN, whereas α3β1 preferentially recognizes LNs.
Integrins are involved in several fundamental cell biological processes. First, and most prominently, they mediate the adhesion of cells to their substrates by providing a physical link between the ECM and the cytoskeleton. Second, integrins can act as signaling receptors that relay information concerning the substrate to the interior of the cell (outside-in signaling), which can, in turn, be interpreted as growth, differentiation, or survival signals2. The binding of integrin to the ECM triggers a series of biochemical signals, such as activation of focal adhesion kinase (FAK) and its downstream effectors, activation of the Ras-extracellular signal-related kinase (ERK) cascade, and activation of PI3-kinase and proteins of the Rho family (Fig. 2). These signals are the same as, or at least substantially overlap with, those activated by the receptor protein tyrosine kinases (RPTKs), which bind to soluble growth factors or cytokines. In fact, an increasing body of evidence suggests that the integrin and the RPTK signals are integrated at cell matrix adhesion sites. The association of integrins with growth factor receptors is indicated by co-clustering and co-precipitation studies3. Not only physical association but also functional cooperation between integrins and growth factor receptors have been demonstrated. The mitogen-activated protein (MAP) kinase pathway provids the best characterized example of this principle, since a number of integrins and growth factor signals converge at multiple points4. Adding soluble mitogens to a suspension of cells triggers the weak or transient activation of the ERK, compared with strong and sustained ERK activation observed in adherent cells. Conversely, RPTKs affect integrin signaling and the assembly of cell matrix junctions, contributing to cell migration.
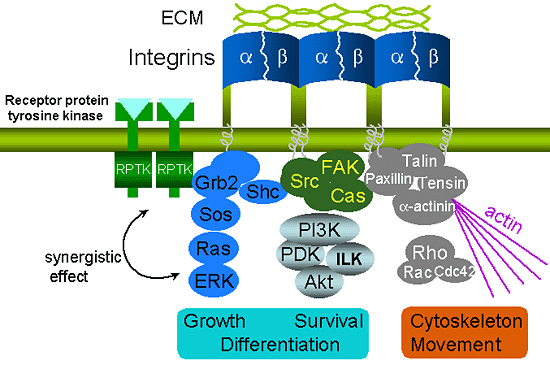
Fig. 2 The association of integrin and ECM can activate several signaling pathways, and subsequently affect cell shape, migration, proliferation and differentiation.
It has also been reported that integrin synergizes with other cell surface receptors such as receptor protein tyrosine kinases (RPTKs) to activate these signaling pathways.
Because integrins differ in their ligand binding specificities, matrix adhesion often determines different signaling. The majority of studies of integrin-mediated signaling events have been performed on cells that adhere to FN through the α5β1 integrin, one of the best characterized integrins, that recognizes the tripeptide sequence, Arg-Gly-Asp. The association between FN and α5β1 integrin is involved in regulating not only cell adhesion and migration but also differentiation and apoptosis5. Endothelial cells proliferate in response to mitogenic growth factors when adhering to FN, whereas they undergo growth arrest when adhering to the α2β1 ligand LN-1. On the other hand, α3β1 integrin-mediated signaling events triggered by cell adhesion to the basement membrane LN-10/11 are quite different from those triggered by adhesion to FN; α3β1 integrin preferentially promotes cell migration6 and prevents apoptosis7. These results suggest that different extracellular matrix components are able to transduce distinct signals through the preferential activation of subsets of multiple integrin-mediated signaling pathways.
These cell surface integrins are all major carriers of N-glycans. Based on the amino acid sequences, both integrins α3β1 and α5β1 contain, respectively, fourteen and twelve potential N-linked glycosylation sites on each of the α and β1 subunits. Determination of the structures of N-linked oligosaccharides on integrins had previously been thought to be tedious work, since the purification of an integrin is difficult, and it is usually obtained in small quantity. However, Takahashi's group developed a sensitive analytical method using different properties of HPLC columns, and overcame this difficulty8. Finally, 35 different oligosaccharide structures have been identified in α5β1 integrin purified from human placenta, 10 of which were neutral, 6 mono-sialyl, 10 di-sialyl, 7 tri-sialyl. The molar ratio of neutral and sialyl oligosaccharides was 20.8% and 77.7%, respectively, whereas high mannose-type oligosaccharides composed only 1.5% of the total.
On the other hand, the structures of oligosaccharides of integrin α3β1 from human ureter epithelium cells (HCV29) were characterized by means of matrix-assisted laser desorption/ionization mass spectrometry9. Similarly, most of the oligosaccharides of α3β1 were complex types with a wide heterogeneity including bi-, tri-, and tetra-antennary sugar chains, while high mannose-type structures were minor components. These results strongly suggest that the N-glycosylation of native integrins is fully processed through the Golgi apparatus. Accumulating evidence indicates that the presence of the appropriate oligosaccharide can ensure the correct folding of a protein as it transverses the endoplasmic reticulum and Goli apparatus, without which it would be misfolded and inactive10. In fact, N-linked high mannose oligosaccharides of pre-integrin β1 serve as a signal for degradation by a ubiquitin ligase complex11.
The N-glycosylation is required for the association of α and β subunits, since a treatment of purified integrin α5β1 with N-glycosidase F resulted in a dissociation of both subunits, and then blocked its binding to FN12. Furthermore, the glycosylation can act as a key regulatory switch for the integrin activation. When human fibroblasts were cultured in the presence of l-deoxymannojirimycin, an inhibitor of α-mannosidase II, which prevents N-linked oligosaccharide processing, immature α5β1 integrin receptors appeared on the cell surface, and FN-dependent adhesion was greatly reduced13. Consistent with this observation, the sialylation on non-reducing ends of N-glycans of α5 and β1 subunits, differentially led to an increase or a decrease in its binding to FN, respectively14, 15. These findings suggest that the interaction of cell-surface integrin α5β1 with FN is dependent on glycosylation and processing status of integrin.
The N-Glycosylation of proteins occurs in the Golgi apparatus by the action of various glycosyltransferases (also called glyco-genes), which are responsible for the synthesis of the huge diversity of complex oligosaccharides16. The manipulation of glycogenes using genetic and biochemical approaches including the induction of gene expression and gene knockout, could be a useful tool for characterizing a specific N-glycan17. N-acetylglucosaminyltransferase III (GnT-III), a pivotal glycosyltransferase, participates in the branching of N-glycans by catalyzing the formation of a unique sugar chain structure, bisecting GlcNAc. The introduction of a bisecting GlcNAc results in the suppression of further processing and the elongation of N-glycans catalyzed by other glycosyltransferases, since they are not able to use the bisected oligosaccharide as a substrate. Thus, GnT-III is generally regarded to be a key glycosyltransferase in N-glycan biosynthetic pathways. It is interesting to note that the metastatic capabilities of B16 mouse melanoma cells are down-regulated by the introduction of the GnT-III gene18. This anti-metastatic effect has been, in part, attributed to the effect of GnT-III on an increase in E-cadherin-mediated homotypic adhesion and the suppression of the phosphorylation of the E-cadherin-β-catenin complex on cell-cell adhesion19, 20. It has also recently been found that the modification of the N-glycans of integrin by GnT-III inhibits its ligand binding ability, subsequently leading to the down-regulation of cell spreading and migration as well as integrin-mediated signaling13. Accordingly, the overexpression of GnT-III inhibits tumor metastasis by at least two mechanisms: an enhancement of cell-cell adhesion and a down-regulation of cell-ECM adhesion (Fig. 3).
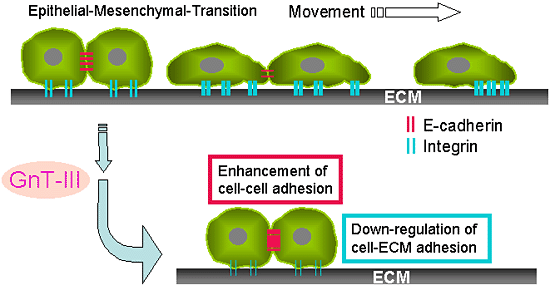
Fig. 3 Cancer metastasis and GnT-III.
A, Epithelia to mesenchymal transition (EMT) correlates with the progression of tumor cells towards invasive phenotypes that mimic late stage tumorigenesis in vivo. B, Possible mechanisms for the suppression of cancer metastasis by the overexpression of GnT-III could be considered: one is an enhancement in cell-cell adhesion by an increase in the stability of E-cadherin on the cell surface and the suppression of the phosphorylation of β-catenin, and the other is the down-regulation of integrin-mediated cell-ECM adhesion.
On the other hand, it has been known that the β1, 6 GlcNAc branching structure acquires the properties of cancer invasion and metastasis21. In fact, β1, 6 GlcNAc branched tri- and tetra-antennary oligosaccharides were found to be significantly increased in α5β1 integrin from NIH3T3 transformed with oncogenic Ras compared with those of wild-type NIH3T322. Similarly, the characterization of carbohydrate moieties of integrin α3β1 from non-metastatic and metastatic human melanoma cell lines showed that β1, 6 GlcNAc branched structures were expressed at high levels in metastatic cells compared with non-metastatic cells23. To explore the possible mechanisms involved in increased N-linked β1,6 GlcNAc branching in metastatic cancer cells, Guo et al. transfected GnT-V cDNA into human HT1080 cells and found that an increase in branched sugar chains inhibits the clustering of integrin α5β1and the organization of F-actin into extended microfilaments in cells plated on FN-coated plates, and subsequently promotes cell migration and invasion24, confirming the hypothesis that the degree of adhesion of cells to their ECM substrate is a critical factor in regulating the rate of cell migration, i.e., migration is maximal under conditions of intermediate levels of cell adhesion25.
As described above, the modulation of the N-glycans of integrins can significantly alter their biological functions including cell spreading, migration as well as signaling transduction. Since the integrins contain multiple potential N-linked glycosylation sites on each α and β subunit, it is essentially important to completely characterize N-glycans on these potential sites for developing a better understanding of the functional roles of glycosylation in regulating physiological cellular signaling including complex formation in microdomain as described26, and pathological processes such as inflammation, infection, cancer, and cancer metastasis as well.