
Chihiro Sato
Graduated from the Department of Biophysics and Biochemistry, Graduate School of Science, University of Tokyo in 1992. Obtained a Ph. D. degree in 1997. From 1997-2001 she worked as a postdoctoral fellowship of the Japan Society for the Promotion of Science for Japanese Junior Scientists in the Dr. Ken Kitajima’s laboratory. From 2001, she has been working at the Department of Applied Biological Sciences, School of Agricultural Sciences, Nagoya University, as an assistant professor. Her recent research focuses on the biological function of the di-, oligo-, and polySia glycotopes.

Ken Kitajima
Graduated from the Department of Biophysics and Biochemistry, Graduate School of Science, University of Tokyo in 1987. Awarded the degree of Doctor of Science. 1987 - 1989: Postdoctoral fellowship of the Japan Society for the Promotion of Science for Japanese Junior Scientists; 1989 - 1996: Assistant Professor at the Graduate School of Science, University of Tokyo; 1996 - 2001: Associate Professor at the Graduate School of Agricultural Sciences, Nagoya University; 2001-present: Associate Professor at the Nagoya University Bioscience Center.
In 1999 awarded the Japanese Society of Carbohydrate Research Award for Young Scientists.
Sialic acids (Sia) are acidic sugars and comprise a family of almost 40 naturally occurring derivatives of N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc) and deaminoneuraminic acid (KDN; 2-keto-3-deoxy-D-glycero-D-galactononurosonic acid) with modification by acetylation, sulfation, methylation, lactylation, and lactonization. In most cases, Sia are located at the non-reducing terminal ends of carbohydrate chains as monomeric forms on glycoproteins and glycolipids and play important roles in ligand-receptor interaction and cell-cell communication.
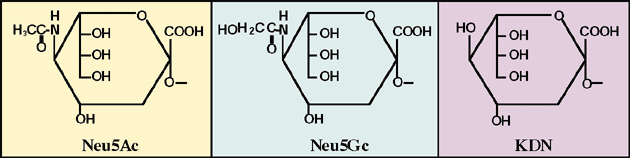
Fig. 1 Structure of sialic acid
In rare cases, Sia are linked to each other to form a polymerized structure, polySia. The polySia glycotope exhibits structural diversity according to differences in the species of Sia (Neu5Ac, Neu5Gc and KDN) and internal Sia linkages (α2→5Oglycolyl, α2→8, α2→9, α2→8/9).
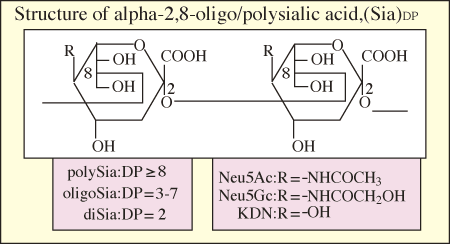
Fig. 2 α2→8-linked sialic acid
Neural cell adhesion molecules (NCAM) have been most thoroughly studied among the polySia-containing glycoproteins. NCAM having an α2→8-linked polySia structure are mainly expressed in embryonic brain. After differentiation into the adult brain, the amount of the polySia structure is largely reduced, while that of NCAM remains unchanged. On the other hand, in adult brain, the polysialylated NCAMs are present in the hippocampus and the hypothalamic nuclei where the ongoing neurogenesis, cell migration, axonal outgrowth and synaptic plasticity are observed. The α2→8-linked polySia is now regarded as an important regulator which prevents strong binding between NCAMs.
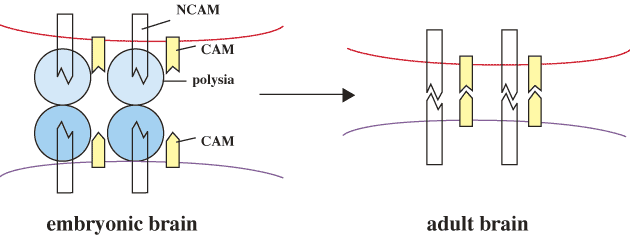
Fig. 3 Function of polySia
The polySia epitope is also expressed in several human cancer cells and widely recognized as an oncodevelopmental antigen and a tumor marker.
Recently, a new class of sialyl groups consisting of di- and oligosialyl (diSia and oligoSia) structures containing up to 7 Sia residues has been shown to occur in glycoproteins more frequently than heretofore recognized using newly developed highly sensitive chemical and immunochemical methods. An α2→8-linked diSia structure in which two Sia resides are tandemly linked to each other is known to be a common structure in gangliosides and plays important biological roles in cell adhesion, differentiation, and signal transduction. On the other hand, the functions of the di/oligoSia on glycoproteins remain for the most part unknown. In this review, we summarize the present status of studies on detection methods, occurrence, biosynthesis and functions of the di/oligoSia of glycoproteins.
Fluorescent C7/C9 Analysis
When an oligo/polymer of α2→8-linked N-acylneuraminic acid (Neu5Acyl) residue is subjected to periodate oxidation, the non-reducing terminal residue is oxidized to the C7 analogue of N-acylneuraminic acid, C7(Neu5Ac) (5-acetoamide-3,5-dideoxy-L-arabino-2-hepturosonic acid), or C7(Neu5Gc) (5-hydroxyacetoamide-3,5-dideoxy-L-arabino-2-hepturosonic acid) from Neu5Ac and Neu5Gc residues, respectively. On the other hand, the internal residues of Neu5Ac (C9(Neu5Ac)) or Neu5Gc(C9(Neu5Gc)) remain intact. Accordingly, the detection of C<9-compounds in the periodate oxidation products strongly suggests the presence of internal sialyl residues or oligomeric structure of α2,8-linked N-acylneuraminic acid therein. Fluorescent labeled C7- and C9-compounds with 1,2-diamino-4,5-methylenedioxibenzene (DMB), which is a-keto acids specific labeling reagent, were identified and quantified by fluorometric high performance liquid chromatography (HPLC). As much as 1 ng of internal sialyl residues of α2→8-linked oligo/polySia chains was detected by this method. This fluorometric C7/C9 analysis was successfully applied to glycoproteins blotted on polyvinylidene fluoride (PVDF) membranes.
Mild Acid Hydrolysis-fluorescent HPLC Analysis
A series of oligo/polymers produced by mild acid hydrolysis of oligo/polySia chain were directly labeled with DMB and analyzed on an anion exchange HPLC. With this method, DMB labeling can be used to detect in glycoconjugates various types of sialyl oligo/polymer, which differ in component Sia species, inter-residue linkages and degree of polymerization (DP). The detection limit of the diSia residues was 13 fmol.
Antibodies that Specifically Recognize Di/oligo/polySia Structures
Antibodies are powerful tools for structural and functional analyses of various glycan structures. However, it is necessary to clearly understand the immunospecificity of the antibodies. We analyzed the immunospecificity of various so-called “anti-polySia” antibodies that had so far been established using as test antigens a series of synthetic neo-di/oligo/polysialoglycolipids with defined degrees of polymerization (DP) and demonstrated that those antibodies could be classified into three groups (Groups I, II, and III) based on the DP and requirement of non-reducing terminal end for antibody recognition. The DP of di/oligo/polySia on unknown samples were able to be predicted using these antibodies.
Group I antibodies are the anti-polySia antibody that recognizes polymeric forms of α2→8-linked Sia with DP 8 or greater. These antibodies recognize the helical conformation formed by the internal region of the extended polySia chains. The non-reducing terminal residues are not involved in recognition by the antibodies. Group II antibodies recognize both oligoSia with DP 2-7 and polySia chains and designated anti-oligo+polySia antibodies. This group of antibodies recognizes the distal portion of the oligo/polySia chains, including the non-reducing terminal residue. Group III antibodies, designated anti-oligoSia antibodies, are reactive with oligoSia with DP 2-4, but not with polySia. These antibodies appear to recognize specific conformations of di- and oligoSia with DP 2-4.
Table Classification of anti-di/oligo/polySia antibodies
|
Name oftd
antibody
|
Animal origin a)
andtd
Ig-type b)
|
Specificity ontd
Sia
|
Specificity ontd
DP
|
| <Group I> anti-polySia antibody | |||
| H.46 | ho, poly, IgM | Neu5Ac | DP≥8 |
| 735 | mo, mono, IgG2a | Neu5Ac | DP≥11 |
| <Group II> anti-oligo+polySia antibody | |||
| 12E3 | mo, mono, IgM | Neu5Ac | DP≥5 |
| OL.28 | mo, mono, IgM | Neu5Ac | DP≥4 |
| 2-4B | mo, mono, IgM | Neu5Gc | DP≥2 |
| kdn8kdn | mo, mono, IgM | KDN | DP≥2 |
| <Gropu III >anti-oligoSia antibody | |||
| S2-566 | mo, mono, IgM | Neu5Ac | DP=2 c) |
| AC1 | mo, mono, IgG3 | Neu5Gc | DP= 2-4 |
Endo-sialidase can serve as a specific probe to detect and selectively modify α2→8-linked polySia chains. Endo-N derived from a bacteriophage K1F catalyzes the following reaction:
(→8Neu5Acylα2→)n-X (n>5) → (→8Neu5Acylα2→)2-4 + (→8Neu5Acylα2→)2-X
Two other types of bacteriophage-derived endo-sialidases whose substrate specificities are different from the Endo-N have been described and used. One is named Endo-NE and requires a minimum chain length of DP≥11 for cleavage. The other is endosialidase from another strain of bacteriophage, and requires oligo/polySia chains with DP≥3 for cleavage.
Exo-sialidases which can cleave specific sialyl linkages are now available. One of them has the ability to cleave the α2,3- and α2,6-linkages of Sia residues specifically. Combinatorial use of this sialidase with the sialidase having broad specificity toward α2,3-, α2,6-, and α2,8-linkages allows us to identify the presence of α2,8-linked di/oligo/polySia chains in sialoglycoconjugates.
The occurrence of the α2→8-linked diNeu5Ac structure in brain and some other tissues was first suggested by Finne and his colleagues in 1977. Later a few diSia-containing glycoproteins were reported to occur in mammals, where the diSia structure was linked to O-linked glycopeptides from chromogranins, a class of related acidic glycoproteins in chromaffin granules from bovine adrenal medulla, O-linked glycan chains of human erythrocyte glycophorin, and N-linked glycans of umbilical cord erythrocyte Band 3. Recently, α2→8-linked diNeu5Ac structures were identified on glycoproteins derived from the ovarian fluid of rainbow trout and α2→8-linked diNeu5Gc structures were found on the rat T cell surface 100kDa glycoprotein. More recently, the authors have demonstrated the presence of the diNeu5Ac-containing glycoproteins in various mammalian cells and tissues. In brain, particular glycoproteins contain the di/oligoNeu5Ac residues, and in serum, bovine fetuin, α2-macroglobulin and adipoQ have been identified as α2→8-linked diSia-containing glycoproteins. The α2→8-linked di/oligoSia structures are also detected in glycoproteins in human lymphoma cells (HL60), human teratocarcinoma cells (PA1), mouse neuroblastoma cells (Neuro2A), mouse myoblastoma cells (C2C12) and mouse preadipocytes (3T3-L1) and, interestingly, these glycotopes on glycoproteins are developmentally regulated. Most recently, integrin α5-subunit on human melanoma has been demonstrated to have α2→8-linked oligoSia structures.
In di- and oligoSia structures, the intersialyl linkage is largely the α2→8-linkage, but an α2→5Oglycolyl- and an α2→9-linkages are also known. The α2→5Oglycolyl-linked di/oligoNeu5Gc structures were found in sea urchin egg glycoproteins, and these di/oligoNeu5Gc chains are sometimes terminated by a 9-O-sulfated Neu5Gc residue at the non-reducing terminus. The α2→9-linked diNeu5Ac structure is only known in glycoproteins of human teratocarcinoma cell line.
Since the expression of α2→8-linked di- and oligoSia structures in glycoproteins is glycoprotein species-specific, cell- and tissue-type specific, developmental stage-dependent, and differentiation-associated, the biosynthesis of these sialyl glycotopes is considered to be strictly regulated. Several groups have cloned cDNAs for several α2,8-sialyltransferases that catalyze the synthesis of the α2,8-linkage, and these enzymes are shown in vitro to be involved in the synthesis of di/oligo/polySia in glycoconjugates. Although two types of enzymes, STX and PST, are both involved in the biosynthesis of the α2,8-linked polySia structure, it remains unelucidated which enzymes are involved in the biosynthesis of the di/oligoSia structures. Many clues to solve this question have been obtained from studies on biosynthesis of polySia chains of NCAM, fish egg PSGP and some other di/oligoSia-containing glycoproteins, and it is now conceivable that ST8Sia III may possibly be responsible for the synthesis of the di/oligoSia structure in many glycoproteins. Notably, trans-sialidase activity with the ability to form the α2→8-linkage has recently been identified in human blood. It is possible that such trans-sialidase activity is involved in the synthesis of the di/oligoSia structures in serum glycoproteins.
Nothing is known about the enzymes that are responsible for the synthesis of α2→8-linkages of KDN, α2→5Oglycolyl Neu5Gc, or α2→9-linkage of Neu5Ac. Very little is known about enzymes that catalyze modifications of the di/oligo/polySia residues by acetylation, lactylation, sulfation, methylation or lactonization, although some pioneering studies have been conducted on the acetylation and methylation of oligo/polySia chains in bacteria and starfish, respectively.
It has been shown that α2→8-linked polySia chains are attached to the embryonic form of NCAM in mammals and their chain lengths dramatically decreased to the oligoSia structure in the adult form of NCAM. Recently, the authors have analyzed developmental changes in oligo/polySia-containing glycoproteins and two interesting observations were made using pig, mouse and rat brains. First, in adult brain, NCAM contains both di- and oligoSia, but not polySia, while in embryonic brain, it is heavily polysialylated. This is a general feature of the expression of di/oligo/polySia structures in vertebrate NCAM. Second, several glycoproteins other than NCAM also have α2→8-linked diSia glycotopes in both embryonic and adult brains, and the amount of these diSia-containing glycoproteins increases in adult brain compared with the embryonic brain. Thus, it is suggested that two distinct events of the expression of the di/oligo/polySia structures proceed during brain development. One is a polySia to di/oligoSia expression shift observed for NCAM, and the other is an increase in diSia expression in several glycoproteins other than NCAM. The polySia structure in NCAM is known to negatively regulate an NCAM-associated cell-cell interaction during neurite outgrowth and synaptogenesis through their repulsive interaction due to their polyanionic property. Therefore, the polySia to di/oligoSia expression shift in NCAM during brain development may switch off the polySia-mediated negative regulation of cell-cell interaction, resulting in enhancement of adhesiveness of neuronal cells due to increased homophilic binding activity of NCAM on the cells. On the other hand, the increase in the di/oligoSia epitope on glycoproteins in adult brain suggests the functional importance of this epitope. For example, the diSia epitope may function as a mediator of cell-cell and cell-ligand interactions thorough the association with some specific recognition proteins. In the case of the α2→8-linked diSia glycotopes on gangliosides, they are well known to play roles in cell adhesion, differentiation, and signal transduction. Most recently, the authors have demonstrated that the diSia glycotope on certain glycoproteins are involved in the process of neurite extension. It is thus suggested that the diSia on glycoproteins may share some biological functions proposed for the gangliosides.
The authors have recently demonstrated that fetuin, α2-macroglobulin (α2M) and adipoQ (Adiponectin, ACRP30) derived are members of diSia-containing glycoproteins in bovine serum. However, only a small set of these glycoprotein molecules (1% of fetuin, 4% of α2M and several % of adipoQ) contain the diSia-containing glycans, and the rest of the molecules usually do not contain such structures. Considering that these glycoproteins are constitutively present in the serum, the diSia-containing species of the glycoproteins may possibly be expressed depending on certain conditions, such as developmental stages and physiological changes. It should be noted that levels of α2M and adipoQ in serum change in response to certain physiological conditions. Furthermore, interestingly, most diSia-containing species of α2M appeared to have a nick scission along the 170kDa-polypeptide chain to form 110 kDa- and 60 kDa-polypeptide chains. It is shown that this type of nick scission usually happens in α2M, when the α2M exhibits protease inhibitor activity during inflammation. Therefore, it can be speculated that diSia structures on the α2M may possibly play some role in inflammation responses. In the same way, the diSia structures on fetuin and adipoQ may modify the physiological roles of these serum glycoproteins. It is noted that sialic acid-binding immunoglobulin type lectins (siglecs) have recently been shown to exist on blood cell surface as well as in serum. Of the so far cloned siglecs (siglec-1 to 11), it is known that siglec-7 and siglec-11 have a high affinity to the diSia structures and siglec-1 and -5 have a low affinity to them. Therefore, it is possible that the diSia-containing glycoproteins are associated with those diSia-recognizing proteins.
Gangliosides are known to be involved in the signal transduction of T cell activation. T-cell proliferation is induced by the stimulation of T-cell receptor, e.g., the treatment of T cells with anti-CD3 antibody results in induction of T cell activation. This anti-CD3 antibody-induced activation is known to be enhanced if CD3 is cross-linked together with CD4 by specific antibodies. Recently, it has been shown that co-treatment of rat T cells with anti-ganglioside antibody AC1 (α2→8-linked diNeu5Gc) and anti-CD4 resulted in a marked induction of the T cell activation, although treatment with just one of these antibodies separately failed to activate the T cells effectively. It is worthy of note here that the AC1 epitope is the common epitope of a glycolipid (Neu5Gc)GD1c and the 100 kDa glycoprotein on the T cells of rat. These results suggest the presence of a novel regulatory pathway of T cell activation, where not only CD4 but the diNeu5Gc glycotopes on the glycolipid and/or the 100 kDa glycoprotein are also involved.
Integrin α5 subunit derived from human melanoma cells has been identified as an oligoSia-containing glycoprotein. Integrin is known as a receptor for fibronectin. Involvement of the oligoSia residue of the integrin α5 subunit in binding to fibronectin has been analyzed using linkage specific sialidases, showing that the oligoSia residue on the integrin is necessary for the binding. It is presumable that the oligoSia may contribute to the formation of an appropriate conformation of integrin α5β1 that has a high affinity to fibronectin. In addition, it has recently been shown that disialylgangliosides (GT1b and GD3) interact with a high mannose-type glycan chain on the α5 subunit of integrin, suggesting the possibility that diSia residues of glycolipids on the epithelial cells regulates the interaction with fibronectin. Considering that the di/oligoSia glycotope is the common epitope of glycolipids and glycoproteins as described above, the di/oligoSia structure may be involved in cell-extracellular matrix interaction.
In rainbow trout ovary and ovarian fluid, several glycoproteins have been shown to contain the di-, oligo-, and polySia glycotopes. The functional importance of the oligo/polySia chains of trout ovary PSGP has been well discussed in relation to genus- and species-specific interaction of sperm and egg and mechanical and antimicrobial protection of developing embryos. The α2→8-linked diSia in the glycoproteins and α2→8-linked oligoKDN chains on KDN-gp in rainbow trout ovarian fluid is considered to be important in sperm-egg interaction as well as in biological defense. It should be noted here that α2→8-linked oligo/polySia chains have relatively high binding activity with calcium ion, which is known to be important for various enzymatic reactions as well as for ligand-receptor binding at fertilization and development.
In sea urchin egg, α2→5Oglycolyl-linked polyNeu5Gc and 9-O-sulfated Neu5Gcα2→5 OglycolylNeu5Gc structures have been shown to occur in egg jelly glycoproteins (polySia-gp) and the 350 kDa sperm binding protein (SBP), respectively. Both glycoproteins had these glycotopes on their O-linked glycan chains. Although the functions of the polySia-gp in egg jelly remain uneluciudated, it has been shown that O-linked glycan chains on the 350 kDa SBP contain the sulfated diNeu5Gc structures and have binding activity to the acrosome reacted sperm at fertilization. In sea urchin sperm, 8-O-sulfated Neu5Acα2→8Neu5Ac is found in gangliosides, and this glycotope-containing ganglioside appears to have the ability to bind with 350 kDa SBP on sea urchin egg.
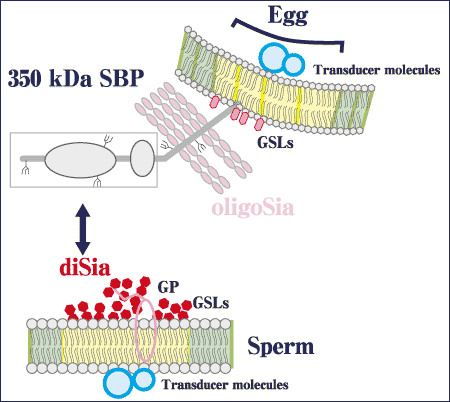
Fig. 4 Functions of the diSia glycotopes in fertilization
Interestingly, the authors have recently demonstrated the presence of this glycotope in glycoproteins as well. Thus, this glycotope is again the common epitope of glycolipids and glycoproteins in sperm, and may be, at least in part, involved in sperm-egg interaction through binding between the egg 350 kDa SBP and the 8-O-sulfated Neu5Acα2→8Neu5Ac glycotopes in ganglioside and/or glycoproteins.
Although there are many functional implications of the di- and oligoSia glycotopes, the following issues will have to be solved before the biological importance of these glycotopes is established. First is the structural determination and identification of carrier proteins of the glycan chains containing these glycotopes. Second is the identification of binding molecules that specifically recognize and bind the di/oligoSia glycotopes. The polySia glycotope on cell has repellent effects on cell-cell interactions through their strong repulsive interaction of negative charges. In contrast, the diSia or oligoSia glycotope has fewer negative charges than polySia, and may possibly be involved in recognition events that are mediated by a specific recognition protein to the di/oligoSia glycotopes. More importantly, such diSia and/or oligoSia recognizing proteins should be examined or described more in the context of biological phenomena. Some glycoproteins have exclusively diSia, and others have both di- and oligoSia. The expression of these glycotopes is strictly regulated in a developmental stage-dependent, differentiation-associated and tissue- and cell-type specific manner. Third is elucidation of how the interaction of the di/oligoSia glycotopes with their receptors on cell surface is transmitted to the signal transduction in cells and subsequently affects the cellular activities. The fourth issue is to understand the significance of the common glycotope between gangliosides and glycoproteins. α2→8-Linked diNeu5Ac structures are now recognized as such common glycotopes in brain glycolipids and glycoproteins on the same cell surface. This is also true of the diNeu5Gc glycotope on rat T cells and the sulfated α2→8-linked diNeu5Ac on sea urchin sperm. In this regard, it is very important to analyze the conformation of common glycotopes between glycoproteins and glycolipids and the membrane domain structure where common glycotopes are colocalized (e.g. lipid rafts).