Hitoshi Sakuraba
Department of Clinical Genetics,The Tokyo Metropolitan Institute of Medical Science
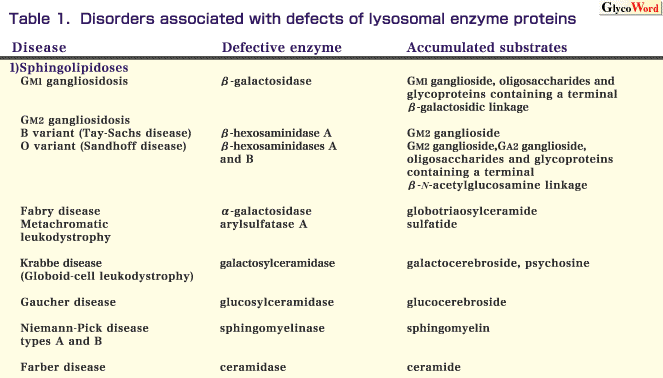
Lysosomes are cytoplasmic vesicles containing many lysosomal enzymes. They are involved in the degradation of cellular substrates including complex carbohydrates and lipids. Genetic defects of lysosomal enzymes and cofactors (activators and stabilizing proteins) cause “lysosomal diseases,” which result in the accumulation of undegraded substrates in lysosomes. Lysosomal diseases comprise a group of more than 30 different disorders, and are classified according to the metabolic pathway affected and the deficient enzymes, as shown in Tables 1-4. The gene causing each disease has been identified for most lysosomal diseases.
Many lysosomal enzymes are exohydrolases acting in sequence, and the terminal residues of their substrates are removed through a stepwise process. Thus, the deficient enzyme activities affect the catabolism of sphingolipids, cholesteryl ester and triglyceride, glycogen, glycoproteins and mucopolysaccharides. These diseases are summarized in Table 1.

1) Sphingolipidoses
Specific defects of the lysosomal enzymes involved in sphingolipid catabolism result in sphingolipidoses.
GM1 gangliosidosis is an autosomal recessive inherited disease caused by beta-galactosidase deficiency. In typical cases, progressive accumulation of GM1 ganglioside and related glycolipids and glycoproteins results in systemic manifestations, including psychomotor retardation, facial dysmorphism, skeletal dysplasia and hepatosplenomegaly.
GM2 gangliosidosis is characterized by excessive accumulation of GM2 ganglioside, particularly in neuronal cells. GM2 ganglioside is hydrolyzed by beta-hexosaminidase A, which is composed of two subunits, alpha and beta. A defect of the alpha-subunit results in deficient activity of beta-hexosaminidase A, and thereby causes an autosomal recessive genetic disease, GM2 gangliosidosis B variant (Tay-Sachs disease). The incidence of this disease is very high in the Ashkenazi Jewish population. A defect of the beta-subunit results in a combined deficiency of beta-hexosaminidases A and B (the latter is composed of only beta-subunits), and thereby causes an autosomal recessive genetic disease, GM2 gangliosidosis O variant (Sandhoff disease). Patients with GM2 gangliosidosis develop progressive psychomotor retardation and macular cherry-red spots.
Fabry disease is an X-linked genetic disease resulting from alpha-galactosidase deficiency. It involves systemic accumulation of globotriaosylceramide, especially in the autonomic nervous system, kidneys and cardiovascular system. Classical hemizygous males with this disease develop pain in the peripheral extremities, angiokeratoma, renal failure and cardiovascular disorders.
Metachromatic leukodystrophy is an autosomal recessive disease caused by arylsulfatase A deficiency. The substrate of this enzyme is cerebroside-3-sulfate (sulfatide), and deficient activity of arylsulfatase A causes the storage of sulfatide in the white matter of the central nervous system and in the peripheral nerves. Patients with the classical form of this disease develop progressive neurodegenerative manifestations.
Krabbe disease (globoid-cell leukodystrophy) is an autosomal recessive neurodegenerative disorder. In this disease, deficient activity of galactosylceramidase (galactocerebrosidase) causes the storage of galactocerebroside. The disease is pathologically characterized by the presence of numerous multinucleated globoid-cells, which are macrophages containing undigested galactocerebroside. It is postulated that the accumulation of psychosine leads to oligodendroglia degeneration.
Gaucher disease is characterized by the accumulation of glucocerebroside caused by deficient activity of glucosylceramidase (glucocerebrosidase). Three types of this disease have been delineated on the basis of the clinical course-type 1, chronic non-neuropathic form; type 2, acute neuropathic form; and type 3, subacute neuropathic form. Hepatosplenomegaly occurs in all forms of the disease. The incidence of type 1 Gaucher disease is very high in Jewish people. Recently, enzyme replacement therapy has been developed for this disease.
Niemann-Pick disease types A and B result from acid sphingomyelinase deficiency. The former is an early-onset severe type associated with a rapidly progressive neurological disorder and hepatosplenomegaly. The latter is a phenotypically heterogeneous disorder, although many patients with this type are diagnosed in infancy or childhood based on the presence of hepatosplenomegaly.
Farber disease is an autosomal recessive disorder associated with acid ceramidase deficiency. The major manifestations of this disease are painful and deformed joints, hoarseness and subcutaneous nodules.
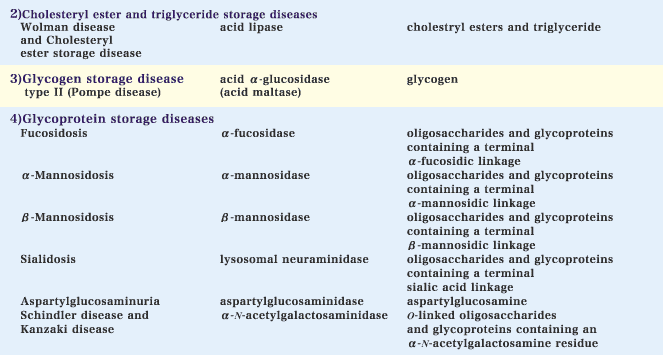
2) Cholesteryl ester and triglyceride storage diseases
Deficient activity of acid lipase results in the accumulation of cholesteryl esters and triglyceride. Genetic acid lipase deficiency includes two clinically different disorders, Wolman disease and cholesteryl ester storage disease. The former is a severe phenotype and fatal before the age of 1. Patients with this type develop hepatosplenomegaly, steatorrhea and adrenal calcification. The latter is phenotypically more benign compared with the former.
3) Glycogen storage disease type II
Glycogen storage disease type II (Pompe disease) is an autosomal recessive disease caused by a deficiency of acid alpha-glucosidase (acid maltase), which is involved in the degradation of glycogen in lysosomes. The classical type is a severe generalized disorder of infancy characterized by cardiomegaly, macroglossia and muscle weakness. The other type is a more slowly progressive disorder with late onset and manifestations limited to skeletal muscle.
4) Glycoprotein storage diseases
Specific deficiencies of the lysosomal enzymes involved in glycoprotein/oligosaccharide catabolism cause glycoprotein storage disorders.
Fucosidosis is an autosomal recessive disorder caused by alpha-fucosidase deficiency. There are phenotypically two subtypes of this disease: a more severe infantile type and a milder type. The manifestations of this disease include neurological deterioration, coarse facies and dysostosis multiplex.
alpha-Mannosidosis is an autosomal recessive disorder resulting from alpha-mannosidase deficiency. Patients with this disease usually develop neurosomatic manifestations including mental deterioration, hepatosplenomegaly and dysostosis multiplex.
beta-Mannosidosis results from a genetic beta-mannosidase deficiency. Most patients with this disease develop mental retardation and facial dysmorphism.
A primary defect of lysosomal neuraminidase (sialidase) causes an autosomal recessive genetic disorder, sialidosis. The disease is clinically divided into two types: sialidosis type 1, characterized by macular cherry-red spots and myoclonus, and sialidosis type 2, distinguished from type 1 by the presence of dysmorphic features besides neurological symptoms and cherry-red spots.
Aspartylglucosaminuria is an autosomal recessive disease caused by a deficiency of aspartylglucosaminidase. This enzyme cleaves the N-acetylglucosamine-asparagine linkages in oligosaccharides and glycoproteins. Loss of this enzyme activity leads to the accumulation of the linkage unit in lysosomes, and patients with this disease develop recurrent infections, mental retardation, coarse facies and skeletal dysplasia. The incidence of the disease is high in the Finnish population.
Schindler and Kanzaki diseases are autosomal recessive disorders resulting from deficient activity of alpha-N-acetylgalactosaminidase. These diseases are clinically heterogeneous. Schindler disease is characterized by a neurodegenerative syndrome resembling that of an infantile type of neuroaxonal dystrophy, such as severe psychomotor retardation, blindness and myoclonic seizures. Kanzaki disease is an adult-onset disorder with mild clinical manifestations including angiokeratoma corporis diffusum and mild intellectual impairment.
5) Mucopolysaccharidoses
Mucopolysaccharidoses are a group of genetic diseases caused by deficiencies of lysosomal enzymes involved in the degradation of glycosaminoglycans. Depending on the defective enzymes, specific undegraded glycosaminoglycans are accumulated in tissues and excreted in the urine (Table 1). The clinical manifestations of mucopolysaccharidoses are heterogeneous, although many of them include coarse facies, dysostosis multiplex and organomegaly. These may be associated with hearing loss, visual disturbance, cardiovascular disorders and mental retardation.
The targeting of lysosomal matrix enzymes from the Golgi apparatus to endosomes/lysosomes is mediated by mannose 6-phosphate receptors. UDP-N-acetylglucosamine: lysosomal enzyme N-acetylglucosamine-1-phosphotransferase catalyzes the first step in the synthesis of mannose 6-phosphate recognition markers on the lysosomal matrix enzyme. A defect of this enzyme results in I-cell disease (mucolipidosis II) and pseudo-Hurler polydystrophy (mucolipidosis III), as shown in Table 2. The former is characterized by early onset, severe psychomotor retardation, coarse facies and dysostosis multiplex. The latter is a milder disorder with later onset. In both diseases, the transport of lysosomal matrix enzymes in cells of mesenchymal origin is defective.

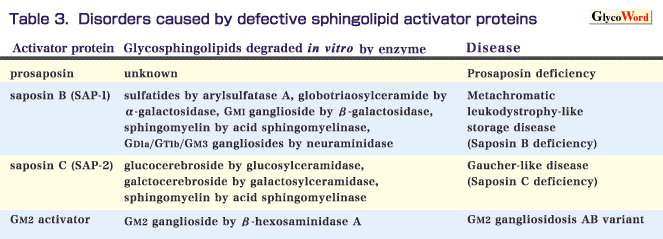
Nonenzymatic glycoproteins are required for the lysosomal degradation of sphingolipids. They are called sphingolipid activator proteins (SAPs). Two genes which encode sphingolipid activator proteins are known. One of them encodes an activator precursor (prosaposin), which is then post-translationaly processed into four mature activator proteins, saposins A to D. The other encodes the GM2 activator. So far, four kinds of sphingolipid activator protein deficiencies have been reported. The functions of the sphingolipid activator proteins and their deficiencies are summarized in Table 3. A defect of prosaposin causes a combined saposin deficiency. This disease is very rare and the clinical findings of the first case were similar to those of Gaucher disease type 2. The clinical findings in most patients with saposin B (SAP-1) deficiency are similar to those of metachromatic leukodystrophy. Patients with saposin C (SAP-2) deficiency show clinical features similar to those of Gaucher disease type 3. The GM2 activator is essentially required for in vivo degradation of GM2 ganglioside by beta-hexosaminidase A, and a genetic defect of it causes neuronal accumulation of GM2 ganglioside. GM2 activator deficiency is called GM2 gangliosidosis AB variant, and the clinical features resemble those of the classic GM2 gangliosidosis B variant or GM2 gangliosidosis O variant.
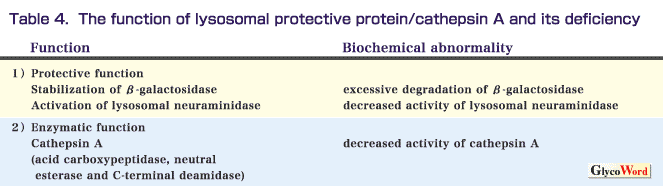
Protective protein/cathepsin A is a multifunctional glycoprotein. It associates with lysosomal neuraminidase and beta-galactosidase to form a multienzymatic complex in lysosomes, activating the former and stabilizing the latter enzyme (protective function). Protective protein/cathepsin A also acts as an acid carboxypeptidase or neutral esterase/deamidase, as shown in Table 4. A defect of protective protein/cathepsin A causes an autosomal recessive disease, galactosialidosis, associated with secondary simultaneous decreases in two glycosidases as well as in cathepsin A. Galactosialidosis is clinically heterogeneous. Most patients develop loss of vision as an initial symptom at age 10-15 years, followed by cerebellar ataxia, myoclonus, cherry-red spots, skeletal dysplasia and angiokeratoma. Severe cases occurring in early infancy have fetal hydrops, edema, renal dysfunction, visceromegaly and coarse facies. Patients with the late infantile form develop visceromegaly, dysostosis multiplex and heart involvement.
Niemann-Pick disease type C is an autosomal recessive disease characterized by defective cellular trafficking of exogeneous cholesterol. The accumulation of unesterified cholesterol, sphingomyelin, phospholipids and glycolipids is prominent in the liver and spleen of patients with this disease (Table 5). The clinical features are heterogeneous, although most patients develop progressive neurological manifestations including vertical supranuclear ophthalmoplegia, ataxia, dystonia and intellectual deterioration.
Multiple sulfatase deficiency is a rare genetic disease associated with a combined deficiency of arylsulfatases A, B and C (Table 5). Patients with this disease exhibit the clinical features of both late infantile metachromatic leukodystrophy and mucopolysaccharidosis.
