
氏名:Kaoru Sakabe
Kaoru Sakabe is a graduate student in the Program of Biochemistry, Cellular, and Molecular Biology at the Johns Hopkins University School of Medicine. She graduated with a B.S. in Biochemistry from the University of North Carolina Chapel Hill before completing a fellowship at the National Institutes of Health.

氏名:Gerald W. Hart
Gerald W. Hart is Director and DeLamar Professor of Biological Chemistry at the Johns Hopkins University School of Medicine. He received his Ph.D. in Developmental Biology at Kansas State University in 1977 and did his post-doctoral work with William J. Lennarz at Johns Hopkins before joining the faculty in the Dept of Biological Chemistry (1979). He was the founding editor-in-chief of Glycobiology.
It has been twenty years since the nucleocytoplasmic protein modification by O-linked β-N-acetylglucosamine (O-GlcNAc) was first described, yet the functions of this ubiquitous post-translational modification remain largely elusive. Challenging the notion at the time that modification by carbohydrates occurs solely on the extracellular surface of the cell or in the endoplasmic reticulum (ER), Golgi, or other sub-cellular organelles, O-GlcNAc was found within the nucleus and the cytoplasm. It has since been demonstrated that O-GlcNAc exists in all multicellular organisms and its misregulation has been linked to diseases such as cancer, Type II diabetes, and Alzheimer’s disease.
O-GlcNAcylation is the enzymatic addition of a single sugar moiety β-N-acetylglucosamine onto the hydroxyl group of Ser and Thr residues of proteins via an O-glycosidic bond. This structure is usually not further elongated like other types of glycosylation. The half-life of O-GlcNAc has been shown to be much shorter than that of the protein backbone, indicating a signaling role in the cell, much like phosphorylation. In fact, many researchers have observed global changes in O-GlcNAc levels in response to stimuli such as mitogen activation, cell cycle, and stress 1,2,3.
Many O-GlcNAcylated proteins have been documented. However, a myriad others remain to be identified. Among those described are RNA polymerase II and transcription factors, tumor suppressors and oncogenes, chromatin and nuclear pore proteins, RNA processing proteins, protein translation regulatory proteins, viral proteins, cytoskeletal proteins, and cytosolic enzymes (Fig. 1)4. All O-GlcNAcylated proteins identified to date are also modified by O-phosphate, hinting that one role of O-GlcNAc may be to complement or modulate phosphorylation. Moreover, sites that are modified by phosphate are often the same or are located within only a few amino acids from the O-GlcNAc site, indicating that these two modifications frequently exist in a reciprocal relationship 5,6,7.
The enzymes involved in the addition and removal of O-GlcNAc have been identified, purified, characterized, and cloned (see ref 8 for extensive review). UDP-GlcNAc:polypeptide Transferase or O-GlcNAc Transferase (OGT) catalyzes the transfer of the N-acetylglucosamine from the activated sugar donor uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), while β-D-N-acetylglucosaminidase or O-GlcNAcase, hydrolyzes the sugar moiety from the protein.
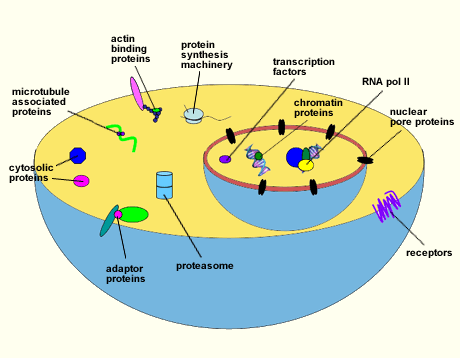
Fig. 1 Diagram illustrating some of the proteins that are O-GlcNAcylated.
A wide variety of proteins have been shown to be modified with O-GlcNAc. This list includes RNA polymerase II and transcription factors, tumor suppressors and oncogenes, chromatin and nuclear pore proteins, RNA processing proteins, protein translation regulatory proteins, cytoskeletal proteins, and cytosolic enzymes.
The activated sugar substrate UDP-GlcNAc is synthesized by the hexosamine biosynthetic pathway (HBP) (Fig. 2). Approximately 2-5% of the glucose entering the cell (depending upon the cell-type) is shunted to this pathway9, which generates amino sugars and activated amino sugars of which UDP-GlcNAc is an end product. UDP-GlcNAc intracellular pools are exquisitely regulated by the nutrient status of the cell, as its precursors are themselves controlled by nucleotide, glucose, amino acid, and fatty acid metabolic pathways. Also, the addition of O-GlcNAc to proteins is highly responsive to UDP-GlcNAc levels over a vast range of concentrations. Furthermore, the peptide specificity of OGT appears to be dependent, in part, on UDP-GlcNAc concentrations10, suggesting that flux through the HBP can control not only OGT activity, but also its targets.
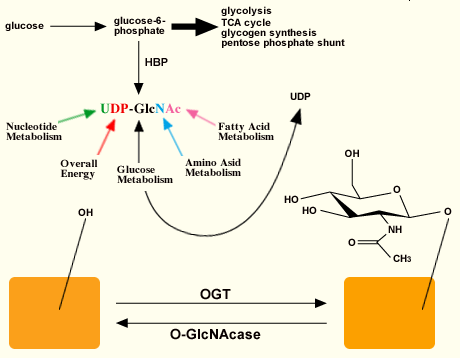
Fig. 2 Relationship between OGT and O-GlcNAcase.
The activated sugar substrate for OGT is synthesized via the hexosamine biosynthetic pathway (HBP). The substrate is sensitive to different metabolic pathways. OGT catalyzes the addition of N-acetylglucosamine to hydroxyl groups of Ser and Thr while O-GlcNAcase hydrolyzes the sugar. These sites of modification can be the same or near sites for phosphorylation.
While there are a multitude of kinases and phosphatases involved in the addition and removal of phosphate, only one OGT catalytic subunit and one O-GlcNAcase have been found. The primary amino acid sequence of OGT has diverged little from C. elegans to humans11. A polypeptide related to OGT at the primary sequence level has not been found in yeast or E. coli. However, the possibility exists that these organisms may have an OGT with different primary structure, or alternatively, they may either not have this type of intracellular glycosylation or they may use a different saccharide besides GlcNAc to accomplish the same functions.
In many tissues, the enzyme is found as a homotrimer consisting of a 110 kDa subunit. However, in some tissues, such as the liver, the predominant form of OGT is a heterotrimer containing two 110 kDa subunits and a 78 kDa subunit12. The 78 kDa subunit appears to result either from proteolysis or from alternate splicing. Additionally, there seems to be a mitochondrially targeted OGT13 arising as an alternate splice variant14. OGT has a bimodal structure; the N-terminal portion of the protein consists of 11.5 to 13 tetratricopeptide repeats (TPR), while the C-terminus contains the catalytic portion. TPR repeats are found in a variety of proteins and they have been shown to be important in protein-protein interactions10. The TPRs in OGT are presumably important not only for substrate recognition, but also for multimerization of the enzyme. The catalytic portion of OGT shows weak homology to glycogen phosphorylase/glycosyl transferase superfamily15.
Fine mapping of the gene localizes OGT to the X chromosome in mice and humans. Of particular note, OGT maps to Xq13.1 in human16, which is also the dystonia Parkinsonism locus. Gene ablation studies in mice indicate the absolute requirement for O-GlcNAc, as the embryonic stem (ES) cells are not viable17.
Although an exact substrate sequence motif has not been found, proline near the site of modification seems to occur frequently10. Interestingly, this observation alludes to a reciprocal relationship between proline-directed phosphorylation and O-GlcNAcylation. In addition, OGT is also modified with both O-GlcNAc and phospho-tyrosine18.
The enzyme that catalyzes the hydrolysis of O-GlcNAc from proteins, O-GlcNAcase, was purified based on its specificity for O-GlcNAcylated peptides and its activity at neutral pH, whereas other glycosidases have an acidic pH optima indicative of their activity residing in lysosomal organelles19. O-GlcNAcase is ubiquitously expressed with an apparent molecular weight of 130 kDa. Interestingly, there is a splice variant of 75 kDa, which is a result of an alternate stop codon. This splice variant lacks the C-terminal portion of the enzyme and lacks catalytic activity20. Although the monomer has catalytic activity, purification of the enzyme from tissue shows that it migrates as a large 340 kDa complex21 indicating associations with a number of proteins in vivo.
O-GlcNAcase is also a bimodal enzyme. The N-terminal portion has loose homology to bacterial hyaluronidases21 while the C-terminus has weak homology to the GCN5-related family of acetyltransferases22. The N-terminus and C-terminus are joined by a linker domain. The gene maps to 10q24, which is also associated with late-onset Alzheimer痴 disease.
O-GlcNAcase is a good substrate for cleavage by Caspase-323, an executioner caspase involved in apoptosis. Although the site of cleavage has not been mapped, cleavage by Caspase-3 does not inhibit O-GlcNAcase activity in vitro.
Protein-Protein Interactions
It is now widely accepted that phosphorylated residues are important structural determinants in protein-protein interactions 24. For example, SH2 domains and 14-3-3 domains bind phosphotyrosine and phosphoserine/threonine residues, respectively. A potential role for O-GlcNAc could be to mediate protein-protein interactions as many proteins that are O-GlcNAcylated are multimeric proteins. For example, YY1 is a zinc finger DNA-binding transcription factor that can be modified by O-GlcNAc. The modification causes dissociation of YY1 from retinoblastoma protein (pRb) allowing it to bind DNA25. O-GlcNAc could also induce binding as it is estimated that approximately 0.1% of the peptides presented by MHC Class I are O-GlcNAcylated. This observation indicates that this post-translational modification is important for recognition by certain T cell receptors26. It is also apparent that O-GlcNAc modification of RNA polymerase II and most transcription factors plays a role in assembly of the pre-initiation complex during the transcription cycle (see below).
Degradation
Another possible regulatory role for O-GlcNAc could be controlling the degradation of proteins. Sequences enriched with Pro, Glu, Ser, and Thr, or PEST sequences, have been proposed to target proteins for rapid degradation27. Interestingly, several O-GlcNAc sites have been mapped to PEST sequences, and it is presumed to protect the protein from degradation. Estrogen receptor β is reciprocally modified by phosphorylation or O-GlcNAcylation at Ser 16. When this site is phosphorylated, the protein is rapidly targeted for degradation, while the O-GlcNAcylated form is degraded much more slowly28. Of note, many of both the regulatory and catalytic subunits comprising the proteasome are modified by O-GlcNAc. O-GlcNAcylation of the proteasome inhibits is ability to degrade certain proteins29. By regulating protein half-life, O-GlcNAcylation can exert a temporal effect on many cellular processes.
Transcription
Virtually every RNA pol II transcription factor studied to date is modified with O-GlcNAc. There is no overlying theme in the specific role of the modification in transcription. In the case of Stat5, the O-GlcNAcylated form binds the coactivator of transcription CBP, activating Stat5 mediated transcription30. However, when Sp1, a ubiquitous transcription factor involved in regulating housekeeping genes, is modified with O-GlcNAc, it is inhibited from interaction with the transcriptional machinery31 at some promoters; however, O-GlcNAcylation of Sp1 increases its activity on other promoters32.
In addition, the C-terminal domain (CTD) of RNA pol II, which is important for interacting with transcription machinery and is indispensable for in vivo function, is also extensively O-GlcNAcylated33. The CTD consists of heptamer repeats that can be multiply modified with O-GlcNAc. It is known that the CTD is also phosphorylated, and that this phosphorylation is absolutely necessary for promoter clearance and elongation. These two modifications are mutually exclusive on RNA polymerase II CTD34. Although the exact role of O-GlcNAc has not been delineated, it may be important for recruitment of RNA pol II to active promoters or formation of the pre-initiation complex. The O-GlcNAcylated form could also act as a readily activatable storage form of RNA pol II31.
Signaling
Transduction of signals from the extracellular surface to the interior, and the adaptations the cell makes in response to these signals dictates both survival and differentiation of the cell. Often these signals are transduced by dynamic post-translational modifications, such as phosphorylation, and proteolysis. It has been shown that mitogenic activation of lymphocytes and cerebellar neurons causes rapid and transient changes in O-GlcNAc levels1,5. These observations indicate that the transitory alterations in O-GlcNAc are needed to effect a concomitant modification in activity of target proteins or gene transcription. It is also clear that O-GlcNAc modulates the insulin signaling pathway (see below). These studies confirm that O-GlcNAc is a dynamic post-translational modification that is involved in signaling.
Cancer
A number of proteins involved in the progression of cells into a cancerous state have been identified as O-GlcNAc modified proteins. Among them are β-catenin, p53, pRb family members, and c-Myc4. In particular, the major O-GlcNAc modification site on c-Myc, Thr58, is a known hot-spot for mutation in Burkitt`s lymphoma and is a major GSK3β phosphorylation site35. In addition, O-GlcNAcase activity is increased in certain cancerous tissues. A recent study comparing different breast cancers with its corresponding normal tissue showed that there was increased O-GlcNAcase activity in the tumor with a corresponding decrease in O-GlcNAc levels36. These observations raise the notion that O-GlcNAc is important in regulation of key phosphorylation events that regulate cellular growth.
Neurodegenerative Disease
Both OGT and O-GlcNAcase map to loci linked to neurodegenerative diseases; the locus for OGT is associated with Parkinson痴 disease, while the locus for O-GlcNAcase is linked with late onset Alzheimer痴 disease. These observations imply that O-GlcNAc may play a role in neurodegenerative diseases. In fact, tau, a microtubule binding protein associated in the pathology of Alzheimer痴 disease, is both phosphorylated and O-GlcNAcylated. Tau in normal brains is extensively O-GlcNAc modified. Hyperphosphorylated tau is found in the aggregates of neurofibrillary tangles linked with Alzheimer痴. It has been postulated that decreasing O-GlcNAc levels in the brain leads to the abnormal phosphorylation of tau37; hence, O-GlcNAc has a protective effect. Beta amyloid precursor protein (APP), neurofilaments, and many synaptic vesicle proteins are also extensively O-GlcNAc modified38,39,40. These recent studies have presented evidence for alterations in O-GlcNAc in neurodegenerative disease in humans.
Diabetes
It is estimated that approximately 177 million people are affected with diabetes worldwide41. Approximately 5-10% of these individuals are Type I diabetic or insulin-dependent, which is an autoimmune disorder that destroys the body痴 ability to synthesize insulin. Type II diabetes constitutes 90-95% of the population with diabetes. This condition is characterized by the desensitization of peripheral tissues to the action of insulin. Studies implicate that increased flux through the HBP is directly linked to insulin resistance 42. This increased flux would correspondingly increase UDP-GlcNAc levels and, hence, O-GlcNAcylation, perhaps linking O-GlcNAc to the induction of insulin resistance. Studies in cultured adipocytes and transgenic mice have shown that increased O-GlcNAc levels directly cause insulin resistance. In these model systems, chemically or genetically increasing O-GlcNAc levels resulted in a Type II diabetic phenotype in which insulin-stimulated glucose uptake was impaired43,44. In a cultured adipocyte system, chemically increasing O-GlcNAc levels inhibited the insulin signaling pathway at the level of PKB/Akt providing a mechanism by which hyperglycemia can induce insulin resistance. Additionally, other studies demonstrating increased O-GlcNAcylation of proteins involved in metabolism and glucose transport, such as glycogen synthase and GLUT445,46, may further implicate O-GlcNAc in the etiology of Type II diabetes both in terms of blockage of insulin signaling and with respect to glucose toxicity.
The emerging studies concerning O-GlcNAc demonstrate that it is an extremely important post-translational modification. It is involved in a number of regulatory events within the cell and misregulation of these events links O-GlcNAc to diseases such as cancer, neurodegeneration, and diabetes.