 |
Keratan sulfate (KS), which is one of the glycosaminoglycans, is an acidic polysaccharide consisting of a linear carbohydrate chain of repeating galactose (Gal) and N-acetylglucosamine (GlcNAc) (also known as poly-N-acetyllactosamine) with sulfate modification (see the part of “Biosynthesis of keratan sulfate and its sulfation steps” in this series). In human, the biosynthesis of KS is mainly carried out by four enzymes, β1,3-N-acetylglucosaminyltransferase 7(β3GnT7), β1,4-galactosyltransferase 4(β4GalT4), carbohydrate sulfotransferase 6 (CHST6, also known as GlcNAc6ST5) and carbohydrate sulfotransferase 1 (CHST1, also known as KSGal6ST) (see “Biosynthesis of keratan sulfate and its sulfation steps”). Gene-deficient mice for the enzymes, except β4GalT4, have been created and analyzed for their phenotypes. It should be noted that the phenotypes found in the gene-deficient mice may not necessarily result from the lack of KS since loss of these enzymes affect not only synthesis of long KS but also short sulfated glycans.
CHST5 in mice is the enzyme equivalent to CHST6 in human, and catalyzes sulfation of GlcNAc (see “Biosynthesis of keratan sulfate and its sulfation steps”). Since inactive mutations on the gene encoding CHST6 in human cause a congenital eye disease, macular corneal dystrophy (MCD) (see “Genetic diseases caused by defect of keratan sulfate biosynthesis”), CHST5-deficient mice were created and compared to the human MCD phenotypes. Homozygotes of CHST5-deficient mice developed and grew as well as wild type mice, and reproduced normally (1). Corneal opacity was not observed in the mutant mice even under the microscopic observation. On the other hand, corneal thickness in the homozygotes was reduced compared to that in wild type and the heterozygotes. Furthermore, electron microscopy and X-ray diffraction analyses revealed that collagen fibrils in the corneal stroma of CHST5-null mice were thinner and interfibrillar distances were closer than that of mice having active CHST5. Collagen fibrils in the CHST5-null cornea were also more disorganized in their alignment than in wild type and heterozygotes (1). In addition to the corneal opacity, thinning of corneal thickness and disorganized collagen fibril alignment have been reported in human MCD (2), indicating that CHST5-null mice share their phenotypes with MCD in human in these respects. Biochemical analysis showed that CHST5-null corneas lacked antigenic carbohydrate structure recognized by the monoclonal antibody 5D4, which binds to highly-sulfated KS structures, indicating defective KS production in the mutant mice (1).
Sulfation of Gal on KS is catalyzed by CHST1 (3). Mutant mice lacking CHST1 activity have been created and analyzed. As seen in CHST5-null mice, CHST1-deficient homozygous mice developed and grew normally and reproduced normally (4). Meanwhile, tissues from CHST1-null mice were completely devoid of highly sulfated KS, indicating that CHST1 is the essential enzyme for Gal sulfation on KS (4,5). CHST1 is present in the lymph nodes and lungs and is thought to act on the production of short sulfated glycans such as sialyl 6'-sulfo-Lewis X and sialyl 6,6'-LacNAc structures in addition to KS. Since the short sulfated glycans are candidate ligands for Siglec-F and L-selection on lymphatic cells, binding of Siglec-F and L-selection on the tissues of CHST1-null mice was analyzed. Contrary to expectations, these proteins bound to the mutant tissues as to wild type tissues, indicating that presence or absence of sulfated Gal does not affect to the binding of Siglec-F and L-selection to the glycan ligands (4,6). Despite of that, infiltration of macrophages and lymphocytes into antigen-sensitized airway was increased in CHST1-null mice, suggesting involvement of Gal sulfation in the immune response (7).
β3GnT7 is an essential enzyme for KS chain elongation. Knockout mice deficient in β3GnT7 production were also created, but no macroscopic abnormalities were observed in developmental and adult stages, which is similar to other KS lacking mutant mice, such as CHST1-null and CHST5-null (8). However, microscopic phenotype of the cornea showed that β3GnT7-null mice had thinner stroma than wild type and heterozygotes. Western blot analysis using an antibody against lumican, one of the major core-proteins carrying KS in the cornea, visualized a great decrease of KS chain length on the proteoglycan in mutant mice, confirming β3GnT7 is crucial for elongation for KS in vivo (8).
Other than the cornea and cartilage, brain is one of the tissues containing KS, but its biosynthesis is more complex than that of cornea. Highly sulfated KS is detected in developing mouse brains, such as embryos and neonates, but not in normal adult brain, which only produces GlcNAc-sulfated KS. However, in the adult central nervous system, neuronal damage such as mechanical injury induces the production of highly sulfated KS at the site of injury (5,9). In normal adult brain, β3GnT7 and CHST5 are responsible for the production of GlcNAc-sulfated KS, a mechanism similar to that in the cornea, and mutant mice lacking these enzymes confirmed indispensable role of them to produce KS (10,11). In developing brains and injured central nervous system, however, GlcNAc sulfation is not dependent on CHST5 but on another carbohydrate sulfotransferase, CHST2 (see “Biosynthesis of keratan sulfate and its sulfation steps” in this series), since CHST2-null mice did not produce KS even though the gene for CHST5 was intact (9) (Figure 1). Again, each mutant mouse lacked production of KS, but no obvious developmental and behavioral abnormalities were observed.
The role of KS for nerve injury was investigated using mutant mice. CHST2-null mice showed faster recovery of injured nerve tissue than wild type mice, indicating that KS production acts in an inhibitory manner on nerve regeneration in lesions of injured tissue (9). On the other hand, CHST2-null mice carrying SOD1G93A transgene, which reads to a phenotypic model of amyotrophic lateral sclerosis (ALS), showed lower survival rate than the transgenic mice with normal CHST2 production, suggesting that the production of KS attenuates pathological symptoms of ALS (12). In the same experiment, survival rate of CHST1-null mice with SOD1G93A transgene was similar to that of the transgenic mice without other genetic alterations, indicating that Gal sulfation on KS is dispensable to show protective effect of the glycan for ALS symptoms (13). On the contrary, J20 mice, which is a mouse model of Alzheimer's disease, with CHST2-null genetic background showed reduced Alzheimer's disease symptoms including decreased amyloid-β deposition, suggesting that induction of KS production may worsen Alzheimer's disease condition (14). Differential contributions of KS to pathological progression were reported in neurodegenerative diseases with different neuroimmune reactivity, thus further studies are needed to establish the KS function on the injured central nervous system.
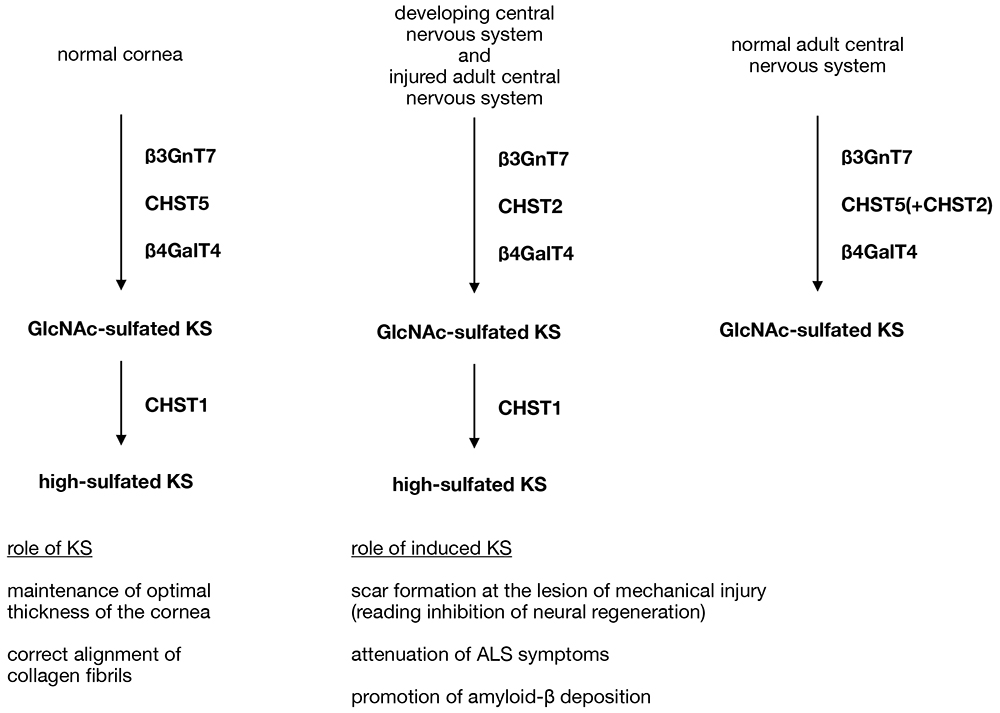
Figure 1. Biosynthetic pathways of KS in mouse
Highly sulfated KS is synthesized in the cornea, cartilage and developing brain in mouse, but the enzyme responsible for GlcNAc sulfation is CHST2 in developing brain whereas it is CHST5 in the cornea and cartilage. In normal adult mouse brain, CHST5 acts to produce GlcNAc-sulfated KS as seen in the cornea, but further sulfation catalyzed by CHST1 does not occur, so highly sulfated KS is absent in the adult brain. However, when adult central nervous system tissues are mechanically or pathologically damaged, induced CHST2 and CHST1 cooperate to produce highly sulfated KS at the site of injury.
|
Tomoya Akama
(Department of Pharmacology, Kansai Medical University)
| References |
| (1) |
Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, Kerr B, Hughes CE, Caterson B, Tanigami A, Nakayama J, Fukada MN, Tano Y, Nishida K, Quantock AJ: Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc. Natl. Acad. Sci. U. S. A. 103, 13333-13338, 2006 |
| (2) |
Quantock AJ, Meek KM, Ridgway AE, Bron AJ, Thonar EJ: Macular corneal dystrophy: reduction in both corneal thickness and collagen interfibrillar spacing. Curr. Eye Res. 9, 393-398, 1990 |
| (3) |
Fukuta M, Inazawa J, Torii T, Tsuzuki K, Shimada E, Habuchi O: Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J. Biol. Chem. 272, 32321-32328, 1997 |
| (4) |
Patnode ML, Yu SY, Cheng CW, Ho MY, Tegesjö L, Sakuma K, Uchimura K, Khoo KH, Kannagi R, Rosen SD: KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin-dependent lymphocyte homing. Glycobiology 23, 381-394, 2013 |
| (5) |
Hoshino H, Foyez T, Ohtake-Niimi S, Takeda-Uchimura Y, Michikawa M, Kadomatsu K, Uchimura K: KSGal6ST is essential for the 6-sulfation of galactose within keratan sulfate in early postnatal brain. J. Histochem. Cytochem. 62, 145-156, 2014 |
| (6) |
Patnode ML, Cheng CW, Chou CC, Singer MS, Elin MS, Uchimura K, Crocker PR, Khoo KH, Rosen SD: Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J. Biol. Chem. 288, 26533-26545, 2013 |
| (7) |
Kumagai T, Kiwamoto T, Brummet ME, Wu F, Aoki K, Zhu Z, Bochner BS, Tiemeyer M: Airway glycomic and allergic inflammatory consequences resulting from keratan sulfate galactose 6-O-sulfotransferase (CHST1) deficiency. Glycobiology 28, 406-417, 2018 |
| (8) |
Littlechild SL, Young RD, Caterson B, Yoshida H, Yamazaki M, Sakimura K, Quantock AJ, Akama TO: Keratan sulfate phenotype in the β-1,3-N-acetylglucosaminyltransferase-7-null mouse cornea. Invest. Ophthalmol. Vis. Sci. 59, 1641-1651, 2018 |
| (9) |
Zhang H, Muramatsu T, Murase A, Yuasa S, Uchimura K, Kadomatsu K: N-Acetylglucosamine 6-O-sulfotransferase-1 is required for brain keratan sulfate biosynthesis and glial scar formation after brain injury. Glycobiology 16, 702-710, 2006 |
| (10) |
Narentuya, Takeda-Uchimura Y, Foyez T, Zhang Z, Akama TO, Yagi H, Kato K, Komatsu Y, Kadomatsu K, Uchimura K: GlcNAc6ST3 is a keratan sulfate sulfotransferase for the protein-tyrosine phosphatase PTPRZ in the adult brain. Sci. Rep. 9, 4387, 2019 |
| (11) |
Takeda-Uchimura Y, Nishitsuji K, Ikezaki M, Akama TO, Ihara Y, Allain F, Uchimura K: Beta3Gn-T7 is a keratan sulfate β1,3 N-acetylglucosaminyltransferase in the adult brain. Front. Neuroanat. 16, 813841, 2022 |
| (12) |
Hirano K, Ohgomori T, Kobayashi K, Tanaka F, Matsumoto T, Natori T, Matsuyama Y, Uchimura K, Sakamoto K, Takeuchi H, Hirakawa A, Suzumura A, Sobue G, Ishiguro N, Imagama S, Kadomatsu K: Ablation of keratan sulfate accelerates early phase pathogenesis of ALS. PLoS One. 8, e66969, 2013 |
| (13) |
Foyez T, Takeda-Uchimura Y, Ishigaki S, Narentuya, Zhang Z, Sobue G, Kadomatsu K, Uchimura K: Microglial keratan sulfate epitope elicits in central nervous tissues of transgenic model mice and patients with amyotrophic lateral sclerosis. Am. J. Pathol. 185, 3053-3065, 2015 |
| (14) |
Zhang Z, Takeda-Uchimura Y, Foyez T, Ohtake-Niimi S, Narentuya, Akatsu H, Nishitsuji K, Michikawa M, Wyss-Coray T, Kadomatsu K, Uchimura K: Deficiency of a sulfotransferase for sialic acid-modified glycans mitigates Alzheimer's pathology. Proc. Natl. Acad. Sci. U. S. A. 114, E2947-E2954, 2017 |
Oct. 24, 2024
|
|---|







