 |
Keratan sulfate (KS) proteoglycans (PGs) are glycoproteins carrying KS, which are sulfated poly-N-acetyllactosamine (polyLacNAc), a linear carbohydrate chain consist of tandemly repeated galactose (Gal) and N-acetylglucosamine (GlcNAc). N-linked, O-linked and O-mannose-linked KS-PGs are mainly found in the cornea, cartilage and brain, respectively (1). Each tissue has different carrier proteins for KS-PGs. Small leucin-rich repeat proteins, such as lumican, keratocan and mimecan, are the major carrier of KS in the cornea. Aggrecan and protein tyrosine phosphatase receptor zeta (phosphacan) are the major core-proteins in cartilage and brain, respectively (1, 2). Characteristics of KS chains in the cornea and cartilage tissues are well analyzed. Corneal KS has longer and more sulfated carbohydrate chains than cartilage KS. Corneal KS has two distinguished regions, highly sulfated region at non-reducing terminal side and GlcNAc-sulfated region at linking side to the core proteins (1). Many non-reducing terminal ends are capped by sialic acid. In mouse, highly sulfated KS is found in embryonic and newborn brains but not in adult brain (3). Instead, GlcNAc-sulfated KS is detected in adult mouse brain (4). Interestingly, highly sulfated KS is locally found at injured area of adult mouse brain and spinal cord (5), suggesting induced expression of enzyme(s) for highly sulfated KS biosynthesis. Sulfation on highly sulfated KS occurs on 6-O positions of hydroxyl group on both Gal and GlcNAc, but the modification is only found on GlcNAc in GlcNAc-sulfated KS (1). There is no report for KS that have multiple sulfation only on Gal, and such KS may not exist in nature. Linkage structures of KS to the core proteins are not the glycosaminoglycan-specific tetrasaccharide structure that is found in chondroitin sulfate- and heparan sulfate-PGs, but commonly found linkage structure in ordinary glycoproteins. From this point of view, KS is not the special glycosaminoglyan chains, but rather a group of sulfated glycoproteins.
Backbone of KS, polyLacNAc, is produced by two families of glycosyltransferases, β1,4-galactosyltransferases (β4GalTs) and β1,3-N-acetylglucosaminyltransferases (β3GnTs). In human and mouse, there are 7 enzymes for β4GalT family, and 8 enzymes for β3GnT family (Fig. 1a, b). Among them, β4GalT4 and β3GnT7 are reported to be responsible enzymes for KS backbone production (6,7). On the other hand, β4GalT1 can synthesize KS in cooperation with β3GnT7 in vitro (8), suggesting β4GalT1 enzyme has some roles on KS backbone elongation.
CHST1 (KSGal6ST) is the responsible enzyme for Gal sulfation (9). CHST3 (chondroitin 6-O sufotransferase) also has activity to produce KS in overexpressed cells (10), however, it is unknown that the enzyme involves in KS synthesis in animals. For GlcNAc-sulfation, there are 5 enzymes (CHST2 (GlcNAc6ST1), CHST4 (GlcNAc6ST2), CHST5 (GlcNAc6ST3), CHST6 (GlcNAc6ST5), CHST7 (GlcNAc6ST4)) in human, and 4 enzymes in mouse (Fig. 1c). CHST5 and CHST6 are highly homologous enzymes to each other as genes for these enzymes (CHST5 and CHST6) were created by gene duplication, and primates possess these two genes but other mammalian and bird genomes only possess either gene, Chst5 or Chst6 (11). Among GlcNAc-sulfation enzymes, CHST6 is the major responsible enzyme for KS sulfation in human, and CHST5 is the one in mouse. From this fact, Chst5 in mouse corresponds to CHST6 in human, and CHST5 is a paralog of CHST6. In adult mouse brain, CHST5 is the major contributor for GlcNAc-sulfated KS production, but CHST2 is responsible for highly sulfated KS synthesis in embryonic and newborn mouse brain, and also at the injured area of adult brain (5).
Study of substrate specificities revealed that CHST6 transfers sulfate on non-reducing terminal GlcNAc but not on internal GlcNAc of polyLacNAc (12). Consistent with this fact, β4GalT4 can transfer Gal on sulfated GlcNAc at non-reducing terminal of the carbohydrate substrate (6), and also β3GnT7 has ability to add GlcNAc on non-reducing terminal Galβ1-4 (SO3--6O) GlcNAc- structure (7). Meanwhile, CHST1 can utilize both non-reducing and internal Gal for sulfation; besides, sialylated and/or sulfated carbohydrates, which are negatively charged, are more preferable substrates than unmodified carbohydrates (13). These data, taken together, indicate that β4GalT4, β3GnT7 and CHST6 cooperatively synthesize GlcNAc-sulfated KS, and then CHST1 acts on production of highly sulfated KS (Fig. 2).
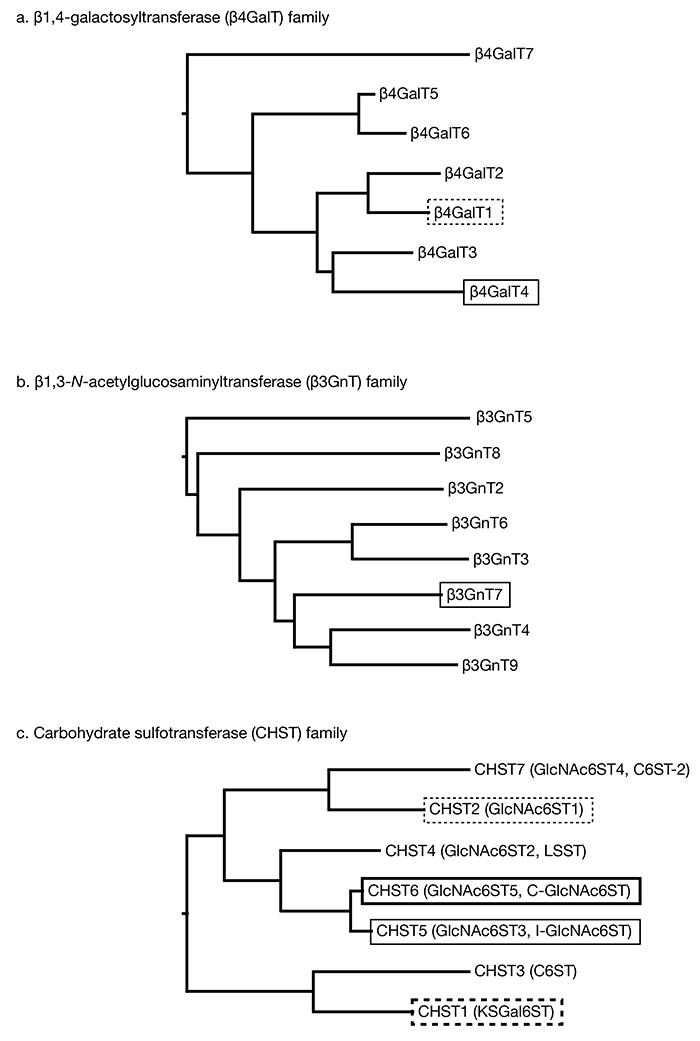
Fig. 1. Phylogenetic trees of enzymes related to KS production
The phylogenetic trees are constructed by Clustal W (GenomeNet at Kyoto University Bioinformatic Center) using amino acid sequences of human enzymes. Boxed enzymes are involved in KS biosynthesis. a. human β1,4-galactosyltransferase family. β4GalT4 (solid box) has been recognized to have the major activity for KS production in animals. β4GalT1 (dashed box) may also have contribution on KS production. b. human β1,3- N-acetylglucosaminyltransferase family. β3GnT7 (solid box) has the major role for KS. c. carbohydrate sulfotransferase family. Only enzymes for 6-O sulftransferases are shown in the tree. We chose the gene name as its protein name and specified its aliases in parentheses. β3GnT1 and β3GnTL1 are not included in the figure, because β3GnT1 (iGnT) has been renamed as β1,4-glucuronyltransferase 1 (β4GAT1), an enzyme involved in α-dystroglycan glycosylation, and β3GnTL1 is a homolog of β4GAT1. Note that CHST5 (solid box) and CHST6 (thick solid box) are encoded in primate genomes, but only either of the enzymes are present in the other species. CHST2 (dashed box) is responsible for highly sulfated KS production in developing mouse brain and injured adult mouse brain. CHST1 (thick dashed box) acts on sulfation of Gal.
|
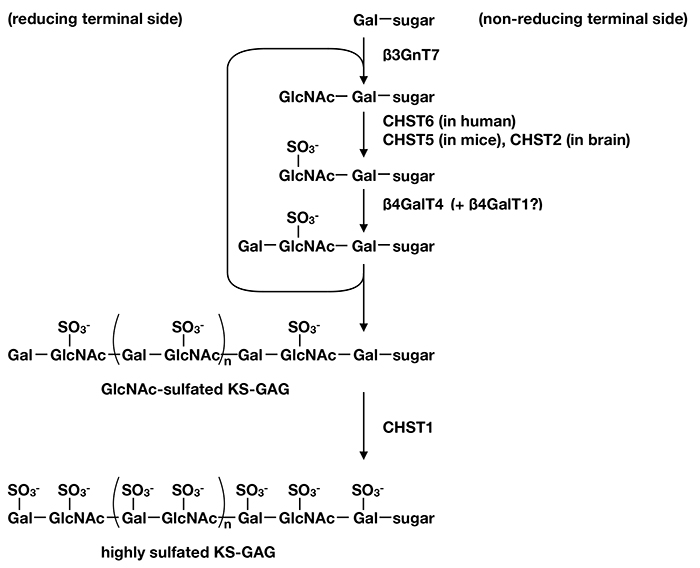
Fig. 2 Biosynthetic pathway of KS
β3GnT7, CHST6 and β4GalT4 cooperatively synthesize GlcNAc-sulfated KS chain, then CHST1 transfers sulfate on Gal to produce highly sulfated KS. In mouse, CHST5 replaces CHST6 as GlcNAc sulfation enzyme. In developing mouse brain and injured adult mouse brain、CHST2 works for KS sulfation. β4GalT1 may act on KS elongation step.
|
Tomoya Akama
(Department of Pharmacology, Kansai Medical University)
| References |
| (1) |
Funderburgh JL: Keratan sulfate biosynthesis. IUBMB Life 54, 187-194, 2002 |
| (2) |
Narentuya, Takeda-Uchimura Y, Foyez T, Zhang Z, Akama TO, Yagi H, Kato K, Komatsu Y, Kadomatsu K, Uchimura K: GlcNAc6ST3 is a keratan sulfate sulfotransferase for the protein-tyrosine phosphatase PTPRZ in the adult brain. Sci. Rep. 9, 4387, 2019 |
| (3) |
Hoshino H, Foyez T, Ohtake-Niimi S, Takeda-Uchimura Y, Michikawa M, Kadomatsu K, Uchimura K: KSGal6ST is essential for the 6-sulfation of galactose within keratan sulfate in early postnatal brain. J. Histochem. Cytochem. 62, 145-156, 2013 |
| (4) |
Takeda-Uchimura Y, Nishitsuji K, Ikezaki M, Akama TO, Ihara Y, Allain F, Uchimura K: Beta3Gn-T7 is a keratan sulfate β1,3 N-acetylglucosaminyltransferase in the adult brain. Front. Neuroanat. 16, 813841, 2022 |
| (5) |
Zhang H, Muramatsu T, Murase A, Yuasa S, Uchimura K, Kadomatsu K: N-Acetylglucosamine 6-O-sulfotransferase-1 is required for brain keratan sulfate biosynthesis and glial scar formation after brain injury. Glycobiology 16, 702-710, 2006 |
| (6) |
Seko A, Dohmae N, Takio K, Yamashita K: β1,4-Galactosyltransferase (β4GalT)-IV is specific for GlcNAc 6-O-sulfate: Beta 4GalT-IV acts on keratan sulfate-related glycans and a precursor glycan of 6-sulfosialyl-Lewis X. J. Biol. Chem. 278, 9150-9158, 2003 |
| (7) |
Seko A, Yamashita K: β1,3-N-Acetylglucosaminyltransferase-7 (β3Gn-T7) acts efficiently on keratan sulfate-related glycans. FEBS Lett. 556, 216-220, 2004 |
| (8) |
Kitayama K, Hayashida Y, Nishida K, Akama TO: Enzymes responsible for synthesis of corneal keratan sulfate glycosaminoglycans. J. Biol. Chem. 282, 30085-30096, 2007 |
| (9) |
Fukuta M, Inazawa J, Torii T, Tsuzuki K, Shimada E, Habuchi O: Molecular cloning and characterization of human keratan sulfate Gal-6-sulfotransferase. J. Biol. Chem. 272, 32321-32328, 1997 |
| (10) |
Fukuta M, Kobayashi Y, Uchimura K, Kimata K, Habuchi O: Molecular cloning and expression of human chondroitin 6-sulfotransferase. Biochim. Biophys. Acta 1399, 57-61, 1998 |
| (11) |
Akama TO, Fukuda MN: Carbohydrate (N-acetylglucosamine 6-O) sulfotransferase 5 and 6 (CHST5,6). In: Handbook of Glycosyltransferases and Related Genes. Taniguchi N, Honke K, Fukuda M, Narimatsu H, Yamaguchi Y, Angata T (editors), Springer Tokyo, Tokyo, Japan, 1005-1014, 2014 |
| (12) |
Akama TO, Misra AK, Hindsgaul O, Fukuda MN: Enzymatic synthesis in vitro of the disulfated disaccharide unit of corneal keratan sulfate. J. Biol. Chem. 277, 42505-42513, 2002 |
| (13) |
Torii T, Fukuta M, Habuchi O: Sulfation of sialyl N-acetyllactosamine oligosaccharides and fetuin oligosaccharides by keratan sulfate Gal-6-sulfotransferase. Glycobiology 10, 203-211, 2000 |
Jun. 15, 2023
|
|---|








