Biosynthesis of Heparan sulfate/Heparin | ||||||||||||||
 |
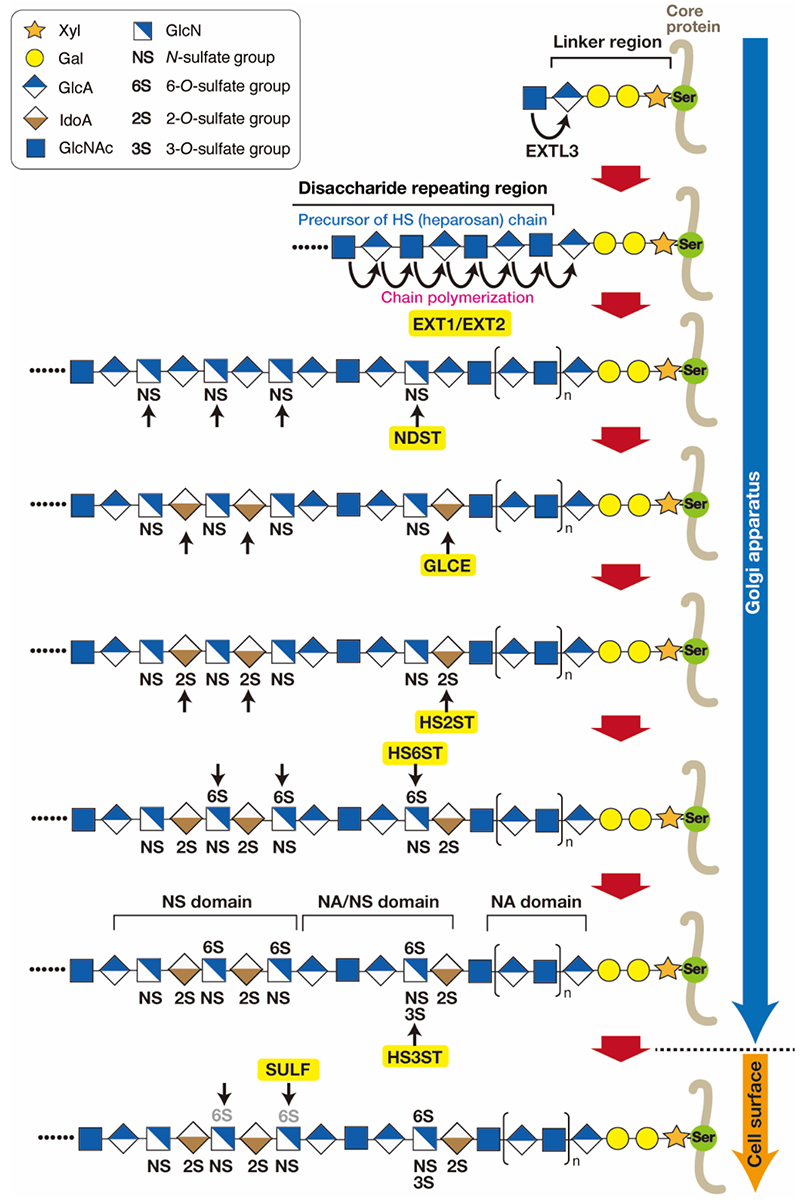
Heparan sulfate (HS) exists ubiquitously as a proteoglycan (PG) and has been implicated in cellular function by interacting with protein ligands, which include a wide variety of growth factors and morphogens (1). Heparin (Hep) has the same polysaccharide backbone structure as HS, but differs in its sulfation degree and expression pattern. Hep is more heavily sulfated than HS, and its expression is restricted to mast cells, where it functions in the storage of granular components, such as histamine and mast cell-specific proteases. Despite the differences, HS and Hep are synthesized through the same biosynthetic pathway. As shown in Figure 1 (2-4), HS chains are covalently bound to serine (Ser) residues in core proteins through a common glycosaminoglycan (GAG)-protein linkage tetrasaccharide structure (GlcAβ1-3Galβ1-3Galβ1-4Xylβ1-O-Ser, where GlcA, Gal, and Xyl are glucuronic acid, galactose, and xylose, respectively) (see the section “Biosynthesis of the common tetrasaccharide sequence in the glycosaminoglycan-protein linker region”). HS biosynthesis is triggered by the transfer of the first N-acetylglucosamine (GlcNAc) residue to the tetrasaccharide linker region, followed by synthesis of the repeating disaccharide region [(-4GlcAβ1-4GlcNAcα1-)n]. The transfer of a GlcNAc residue to the tetrasaccharide linker is catalyzed by exostosin-like 3 (EXTL3) (5), followed by further chain elongation, primarily catalyzed by a hetero-oligomeric complex formed by two homologous HS polymerases, EXT1 and EXT2. During polymerization, precursors of HS and heparosan chains undergo a series of processing reaction by numerous HS-modifying enzymes, including N-deacetylase/N-sulfotransferases (NDSTs), ᴅ-glucuronyl C5-epimerase (GLCE), and different O-sulfotransferases (HS2ST, HS6STs, HS3STs). Every sulfotransferase transfers a sulfate group from 3’-phosphoadenosine 5’-phosphosulfate (PAPS) to the defined position of sugar residues. Finally, the HS structure is modified post-synthetically through the action of plasma membrane-bound 6-O-endosulfatases SULF1 and SULF2 and heparanases (HPAs) (4). Specific sulfation patterns are recognized by a variety of proteins and their interactions are thought to be involved in the regulation of physiological functions. As described above, the initial product of chain polymerization is the polysaccharide precursor heparosan. Subsequently, GlcNAc residues in the repeating unit are partially converted to N-sulfated glucosamine (GlcNS) by NDSTs. The GlcA residues adjacent to these GlcNS units are frequently epimerized to form iduronate (IdoA). The resulting GlcNS/IdoA-rich domains (NS domains) are modified by O-sulfotransferases. A significant proportion of the heparosan chain fails to be modified, and thus, an unmodified stretch consisting of GlcA-GlcNAc disaccharide units (NA domains) still exists in the mature HS chain. Thus, HS chains feature three types of structural domains: NA domains (stretches of unmodified N-acetylated disaccharide units), NS domains (clusters of N-sulfated disaccharide units), and mixed or NA/NS domains composed of alternating N-sulfated and N-acetylated disaccharides (2). NS domains are interspersed with NA domains, and mixed domains are located in the transition zone between the NS and NA domains. Most proteins for which an HS-binding sequence has been identified interact with the NS domains. NA domains are thought to act in space and correctly arrange the NS domains within the HS chain.
Satomi Nadanaka / Hiroshi Kitagawa
|
|||||||||||||
| References | |
|---|---|
| (1) | Bishop JR, Schuksz M, and Esko JD: Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030-1037, 2007 |
| (2) | Esko JD, and Lindahl U: Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169-173, 2001 |
| (3) | Huang YF, Mizumoto S, and Fujita M: Novel insight into glycosaminoglycan biosynthesis based on gene expression profiles. Front. Cell Dev. Biol. 9, 709018, 2021 |
| (4) | Marques C, Reis CA, Vives RR, and Magalhaes A: Heparan sulfate biosynthesis and sulfation profiles as modulators of cancer signalling and progression. Front. Oncol. 11, 778752, 2021 |
| (5) | Nadanaka S, and Kitagawa H: Heparan sulphate biosynthesis and disease. J. Biochem. 144, 7-14, 2008 |
Dec. 13, 2023